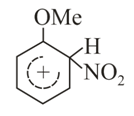
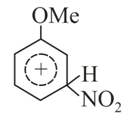
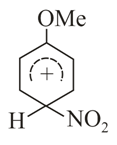
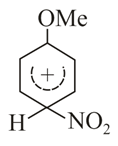
Structures of complex formed during nitration of Anisole would be

Important Points to Remember in Chapter -1 - Hydrocarbons from Embibe Experts Gamma Question Bank for Engineering Chemistry Solutions
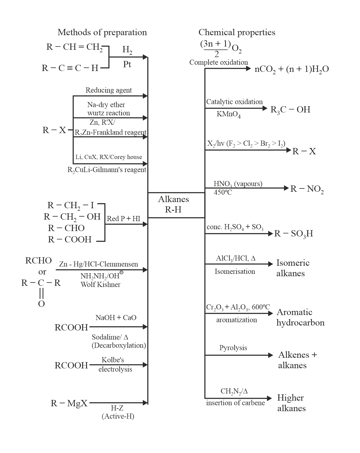
1. Alkanes preparation methods and chemical properties:
2. Physical properties of alkanes:
(i) Boiling Point :
(a) increases with the increase in the molecular mass.
(b) Among the isomers, straight chain alkanes have higher than branched chain alkanes.
(ii) Melting Point :
(a) The melting points do not show a regular variation with an increase in the molecular size.
(b) The even number members have higher as compared to the next alkanes with an odd number of carbon atoms (ALTERATION EFFECT).
(iii) Solubility:
They are soluble in non-polar solvents, and hence, insoluble in polar solvents such as water.
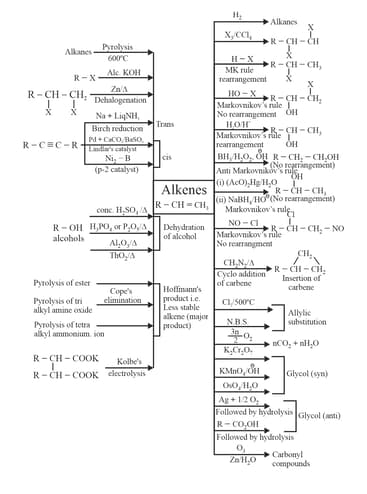
3. Alkenes preparation methods and chemical properties:
4. Physical properties of alkenes:
(i) Solubility: They are insoluble in water but soluble in organic solvents.
(ii) Boiling point: The boiling point of cis alkenes is usually higher than the corresponding trans-alkenes.
(iii) Melting point: The melting point of trans-alkenes is usually greater than cis alkenes.
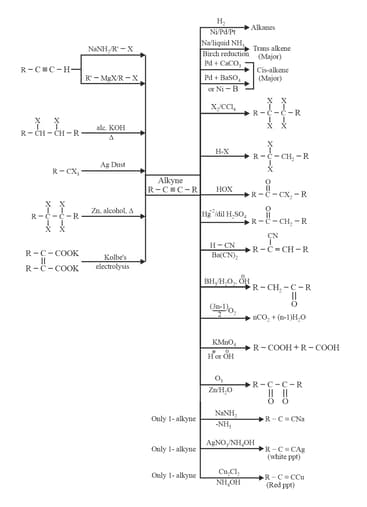
5. Alkynes preparation methods and chemical properties:
6. Aromatic hydrocarbons:
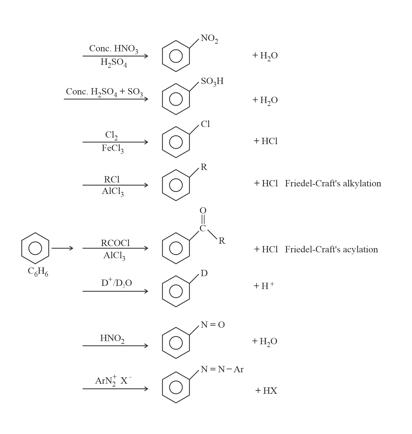
(i) Electrophilic aromatic substitution:
(a) Bromination of Benzene:
Bromination follows the general mechanism for electrophilic aromatic substitution. Bromine itself is not sufficiently electrophilic to react with benzene, but a strong Lewis acid such as , catalyses the reaction.
Step : Formation of a stronger electrophile.
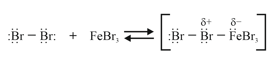
Step : Electrophilic attack and the formation of the sigma complex.
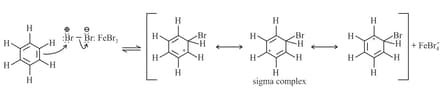
Step : Loss of a proton gives the products.
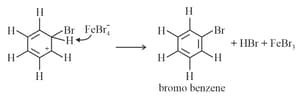
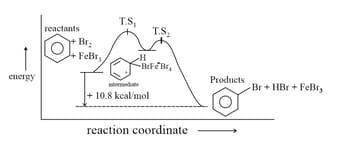
(b) Nitration:
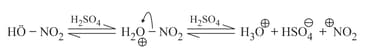
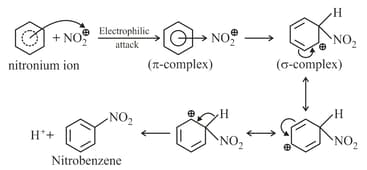
(c) Sulphonation:
The electrophilic reagent, , attacks the benzene ring to form the intermediate carbocation.
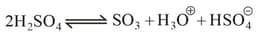
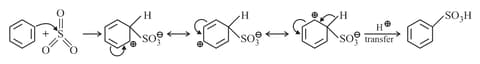
(d) Friedel Craft reaction:
Alkylation mechanism
(i) 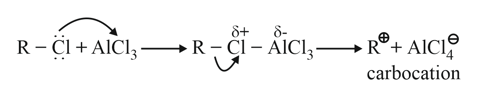
(ii)
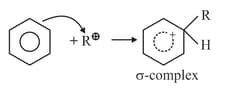
(iii) 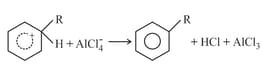
Acylation mechanism
Acylation of benzene may be brought about with acid chlorides or anhydrides in the presence of Lewis acids.
Step : Formation of an acylium ion.
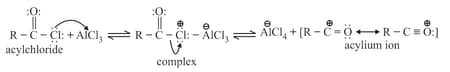
Step : Electrophilic attack.
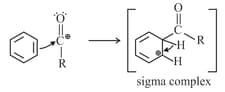
Step : Loss of a proton. Complexation of the product.
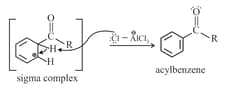
For example:
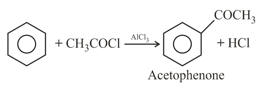
Note: Friedel - Crafts acylation is generally free from rearrangements and multiple substitutions. They do not go on strongly deactivated rings.
7. Chemical Reactions of Benzene:
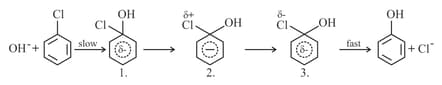
8. Nucleophilic Aromatic Substitution:
The reaction is a second order in which nucleophilic substitution occurs on the benzene ring. It is generally accepted that the reaction proceeds via an intermediate -complex.