Suggest, using ideas of structure and bonding, why: graphene is a good conductor of electricity

Important Questions on States of Matter
Suggest, using ideas of structure and bonding, why:
buckminsterfullerene, C60, is relatively soft.
Four types of structure are:
giant molecular
giant ionic
giant metallic
simple molecular
Give two examples of a giant ionic structure and two examples of a simple molecular structure.
Four types of structure are:
giant molecular
giant ionic
giant metallic
simple molecular
Explain why substances with giant ionic structures are often brittle, but metallic structures are malleable.
Four types of structure are:
giant molecular
giant ionic
giant metallic
simple molecular
Explain why giant molecular structures have higher melting points than simple molecular structures.
Four types of structure are:
giant molecular
giant ionic
giant metallic
simple molecular
Diamond and graphite are two forms of carbon with giant molecular structures. Explain why graphite conducts electricity but diamond does not.
The structures of carbon dioxide and silicon dioxide are shown in the diagram below.
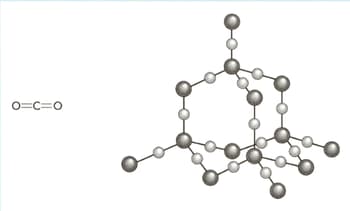
Use your knowledge of structure and bonding to explain the following:
carbon dioxide is a gas at room temperature
The structures of carbon dioxide and silicon dioxide are shown in the diagram below.
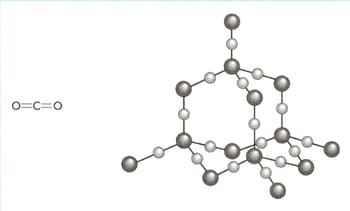
Use your knowledge of structure and bonding to explain the following:
silicon(IV) oxide is a solid with a high melting point
The structures of carbon dioxide and silicon dioxide are shown in the diagram below.
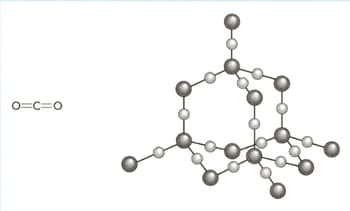
Use your knowledge of structure and bonding to explain the following:
neither carbon dioxide nor silicon(IV) oxide conducts electricity.
