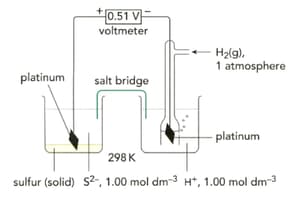
Suggest why aqueous silver nitrate is not used in a salt bridge when connecting a half-cell containing and to another half-cell.

Important Questions on Electrochemistry
Write half-equation for the reaction taking place in the half-cell below. Write the equation as a reduction (electrons on the left-hand side of the equation).
Write half-equation for the reaction taking place in the half-cell below. Write the equation as a reduction (electrons on the left hand side of the equation).
Write half-equation for the reaction taking place in the half-cell below. Write the equation as a reduction (electrons on the left hand side of the equation).
(make sure that you balance the equation)
Write half-equation for the reaction taking place in the half-cell below. Write the equation as a reduction (electrons on the left hand side of the equation).
(make sure that you balance the equation)
Write half-equations for the three reactions taking place in the half-cells shown on the left in the figure.
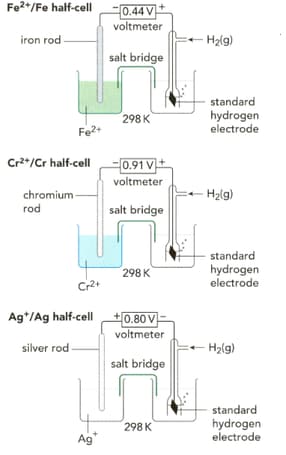
What are the standard electrode potentials for the half-cell reactions shown on the left in the figure?
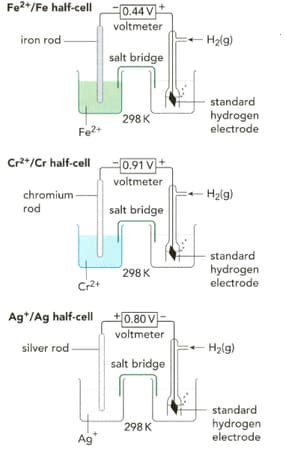
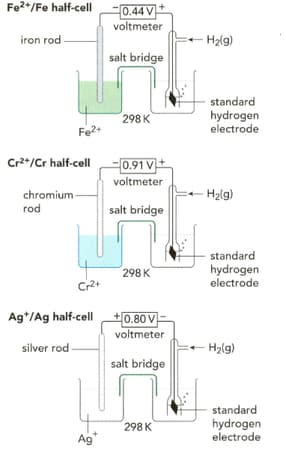
Look at Figure given below. Write a half-equation for the half-cell on the left-hand side.