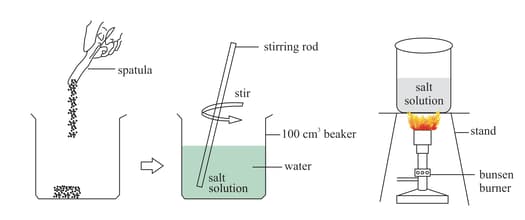
Take three beakers and fill them with water. Add two spoons of salt to each of them. Place the first beaker undisturbed, stir the second and warm the third beaker. What do you observe from the above three situations?
(a) Which method allows the solute to dissolve in the solvent easily?
(b) If you increase the temperature of the third beaker, what will happen?
(c) Repeat the activity by using salt crystals instead of salt powder. What change do you observe


Important Questions on Is Matter Around us Pure?
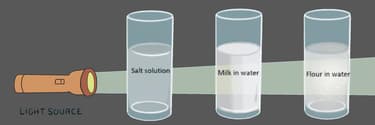
Raju's teacher gave him three distinct substances: common salt, milk, and flour, and he was asked to mix these substances with water. Now help Raju to figure out the kind of mixture formed in each case. Also help him identify the mixture which shows Tyndall effect in the presence of a light source.
Tanishka has been given a chart shown below with three mixtures and related methods of separation. She gave some reasoning for the validation of the methods used which are also recorded in the table.

| Investigation | Mixture | Method of separation | Student's scientific reasoning |
| A | Fabric dye | Paper chromatography | Individual components of liquid mixtures can be separated using the process of chromatography. |
| B | Activated carbon and ethanol | Simple distillation/Evaporation | Simple distillation will result in the removal of water, leaving carbon as a residue. |
| C | Benzene (non-polar) and water (polar) | Filtration | As these two different liquids are not miscible, the denser liquid will be trapped by the filter paper while the other liquid will pass through and can be separated. |
Which of Tanishka's reasoning is/are wrong and if the mentioned methods are wrong then what is the appropriate method for the given mixtures?
Karan's grandmother was showing mild symptoms like cough, fever and tiredness. On consulting with their family doctor, they were advised to observe the temperature change after every two hours for a period of one week. This responsibility was given to Karan and during this task, he noticed that a liquid A raised in the thermometer tube, and he noted the temperature change as advised by the doctor.

Karan would accompany his father for getting the medicines and grocery items. One day, his father stopped the bike at a petrol station on their way home, and he saw that two fuel containers were filling the underground tank at the petrol station. The curious Karan then asked his father whether the two fuel containers contained different liquids, to which he was told that the liquid in one of the containers is petrol and the liquid (liquid B) in the second container is used to make the fuel more efficient.

The next day, the doctor examined the grandmother's throat and found some swelling around the tonsils that has caused infection and fever. He gave a liquid C to her for gargle and advised her to use this thrice a day. It has an anti-inflammatory property.
Karan was curious to know about the three liquids and manages a sample of all the three liquids.
He then started heating the three of them and found that A and B vaporises completely but C leaves a residue after vaporisation. This residue D was similar to the substance in his kitchen that his mother uses for cooking food. The vapours E from liquid C has turned anhydrous copper sulphate to blue.

Help Karan know which liquid is an element, mixture and which is a compound. Also identify D and E.
Tanush and Sanvi were conducting an experiment in the laboratory. Tanush brought a reagent bottle filled with clear, colourless liquid with a petroleum-like odour. He showed this bottle to Sanvi. By seeing and smelling the reagent, Sanvi told Tanush that it was benzene. Tanush went to the chemical chamber again and brought a plastic bottle filled with light yellow liquid and showed it to Sanvi. By smelling it and seeing that plastic bottle, Sanvi suggested that it might be diesel.
Tanush surprisingly took a beaker and poured half of benzene and half of diesel into it, and mixed them well. They saw that the liquids got mixed, and now they could not separate it. They both then approached their teacher and told him everything. So, the teacher suggested a method to separate these two liquids. Can you suggest how to separate a mixture containing benzene and diesel (the difference in their boiling points is more than )?

Lohit, Navika, Naveen, Shilpa, and Abhishek went to an industry visit with their teacher. They went to the chemical industry and visited all the departments in the industry. At last, they went to the testing department, where a group of chemists were testing the newly produced chemicals. They saw that one of the chemists was doing a test by dissolving some chemicals in the water at different temperatures. They asked their teacher about this test, and they requested their teacher to perform this experiment at their school laboratory.

The next day in school, they took three chemicals and performed solubility tests at different temperatures, and created this tabular column.
Table: Variation of solubility with temperature in grams per grams of water.
| Salt | C | C | C | C | C | C |
The teacher then asked them if a solution containing of water and of was heated to , how many additional grams of must be added to prepare a saturated solution at ?
Suhana wanted to separate the mixture of dyes constituting a sample of ink. He marked a line by the ink on the filter paper and placed the filter paper in a glass containing water as shown in the image. The filter paper was removed when the water moved near the top of the filter paper.
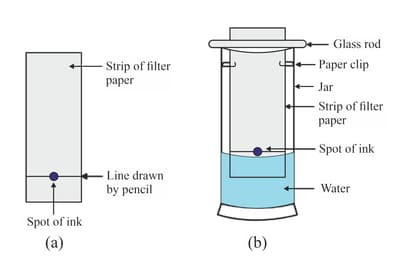
(i) What would you expect to see, if the ink contains three different coloured components?
(ii) Name the technique used by the child.
(iii) Suggest one more application of this technique.
In , English chemist Henry Cavendish isolated hydrogen, which he called "inflammable air". Cavendish discovered hydrogen as a colourless, odourless gas that burns and can form an explosive mixture with air. He published a paper in , on the production of water by burning inflammable air in dephlogisticated air. This experiment simply found that water was condensed from the air through the burning of hydrogen.

Observe the below-given statements and identify which of the statements are the facts.
(P) Dephlogisticated air is in fact oxygen which has an elementary nature.
(Q) Inflammable air is a compound that burns to give another compound water.
(R) Water is a compound that has a fixed ratio of both the air involved in its formation.
(S) Inflammable air on burning breaks into its constituent and as a result water is produced.
Avinash and Pratiksha were doing experiments in a chemistry lab. Avinash took of water at room temperature in a beaker and dissolved a little solid S in it by stirring to obtain a solution X. Then Pratiksha started adding more and more solid S to the solution with constant stirring while keeping the temperature of the solution constant at . At the same time, Pratiksha and Avinash observed that no more solid dissolves in water and, at the same time, some solid is also left undissolved at the bottom of the beaker. So, they separated the contents of the beaker by filtering them through filter paper, and they got solution Y in the form of a filtrate.

Please answer the below question by understanding the information given above.
(a) What type of solution is X?
(b) What type of solution is Y?
(c) What will you observe if the solution Y at is cooled down to by keeping the beaker in crushed ice? Give a reason for this observation.
(d) Give the term used to denote "the amount of solids dissolved in a given water".
