MEDIUM
Earn 100
The 44th Amendment Act of 1978 is associated with which of the following?
(a)Lowering of voting age from 21 to 18 years
(b)Restoration of the Supreme Court's power
(c)Introduction of the Anti-defection law
(d)Reservation of seats for women in Panchayats
50% studentsanswered this correctly
Important Questions on Laws of Motion
MEDIUM
MEDIUM
EASY
If the kinetic energy of a particle of mass performing uniform circular motion in a circle of radius is find the acceleration of the particle.
EASY
MEDIUM
EASY
EASY
Two particles are moving on two concentric circles of radii and with equal angular speed At their positions and direction of motion are shown in the figure:
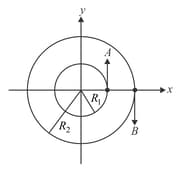
The relative velocity at is given by:
EASY
EASY
EASY
EASY
EASY
MEDIUM
EASY
EASY
EASY
MEDIUM
MEDIUM
EASY

