EASY
Earn 100
The bond angle in alcohols is slightly less than the tetrahedral angle whereas the bond angle in ether is slightly greater because
(a)of repulsion between the two bulky groups
(b) atom in both alcohols and ethers is -hybridised
(c)lone pair-lone pair repulsion is greater than bond pair-bond pair repulsion
(d)None of these
50% studentsanswered this correctly
Important Questions on Alcohols, Phenols and Ethers
EASY
HARD
MEDIUM
MEDIUM
HARD
MEDIUM
MEDIUM
HARD
MEDIUM
MEDIUM
Why ether is insoluble in water?
MEDIUM
MEDIUM
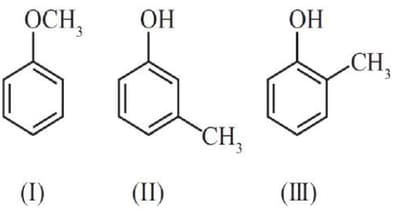
HARD
MEDIUM
An ideal gas in a closed container is slowly heated. As its temperature increases, which of the following statements are true ?
(A) the mean free path of the molecules decreases
(B) the mean collision time between the molecules decreases.
(C) the mean free path remains unchanged.
(D) the mean collision time remains unchanged.
HARD
MEDIUM
MEDIUM
HARD
MEDIUM

