EASY
Upper Secondary: IGCSE
IMPORTANT
Earn 100
The Group metals show a trend in melting points. What does that mean?

Important Questions on The Periodic Table
EASY
Upper Secondary: IGCSE
IMPORTANT
MEDIUM
Upper Secondary: IGCSE
IMPORTANT
One measurement in the table shown below does not fit the trend. Identify the measurement and give the reason for the same. 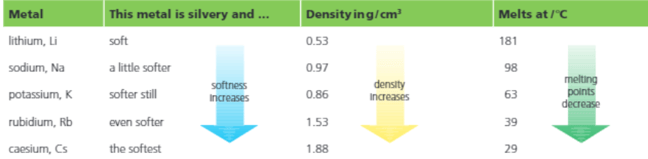
EASY
Upper Secondary: IGCSE
IMPORTANT
EASY
Upper Secondary: IGCSE
IMPORTANT
EASY
Upper Secondary: IGCSE
IMPORTANT
EASY
Upper Secondary: IGCSE
IMPORTANT
EASY
Upper Secondary: IGCSE
IMPORTANT
EASY
Upper Secondary: IGCSE
IMPORTANT
