MEDIUM
JEE Advanced
IMPORTANT
Earn 100
The MO electronic configuration of can be represented as follows:
Following conclusion can be drawn:
(I) It is excited state electronic configuration of
(II) It is more stable state than the ground state of molecule
III) Bond order of in excited state is one
IV) It is more likely to dissociate into two X-atoms in ground state than in excited state
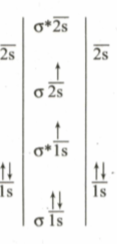
Which of the above conclusions are correct from given MO diagrams?
(I) It is excited state electronic configuration of
(II) It is more stable state than the ground state of molecule
III) Bond order of in excited state is one
IV) It is more likely to dissociate into two X-atoms in ground state than in excited state

(a)(I) and (II)
(b)(II), (III) and (IV)
(c)(I),(III) and (IV)
(d)(I), (II), (III) and (IV)
50% studentsanswered this correctly

Important Questions on Chemical Bonding and Molecular Structure
MEDIUM
JEE Advanced
IMPORTANT
A simplified application of MO theory to the hypothetical 'molecule' would give its bond order as:
EASY
JEE Advanced
IMPORTANT
Which have a non-integral bond order?
MEDIUM
JEE Advanced
IMPORTANT
Which of the following facts regarding change in the bond length is correct?
HARD
JEE Advanced
IMPORTANT
Which of the following orders is correct for the bond dissociation energy of and
MEDIUM
JEE Advanced
IMPORTANT
The nodal plane in the -bond of ethene is located in:
MEDIUM
JEE Advanced
IMPORTANT
Among the following, the paramagnetic compound is:
EASY
JEE Advanced
IMPORTANT
The diatomic molecule exists in the gas phase. The energy levels of and atoms are shown in the figure. Assume that the molecular axis of molecule is -axis.

Predict which of the following is correct about molecule?
MEDIUM
JEE Advanced
IMPORTANT
Which of the following molecular/atomic orbitals have gerade symmetry or centre of symmetry?
