The Plot of -metric titration of weak base vs strong acid looks like
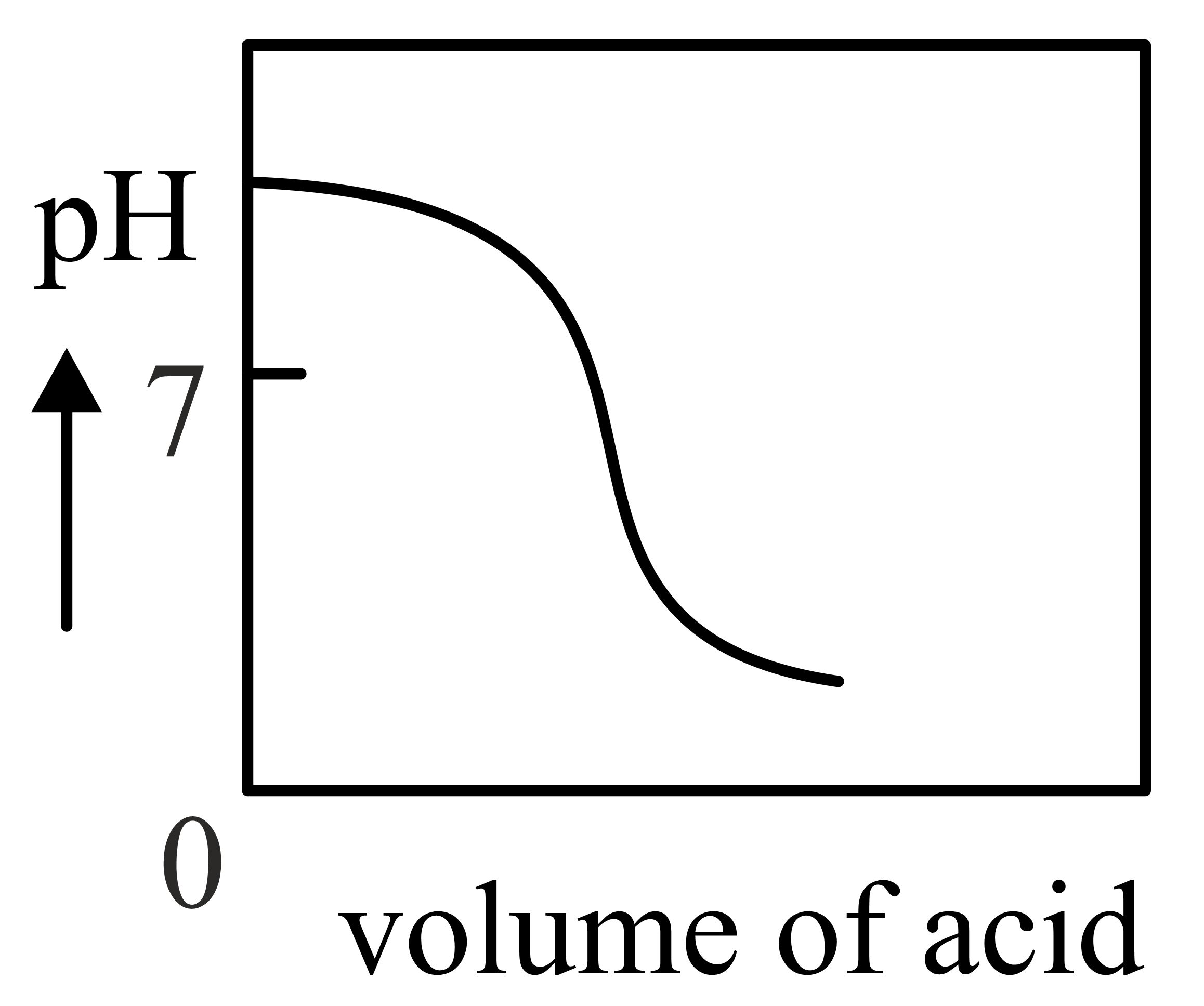
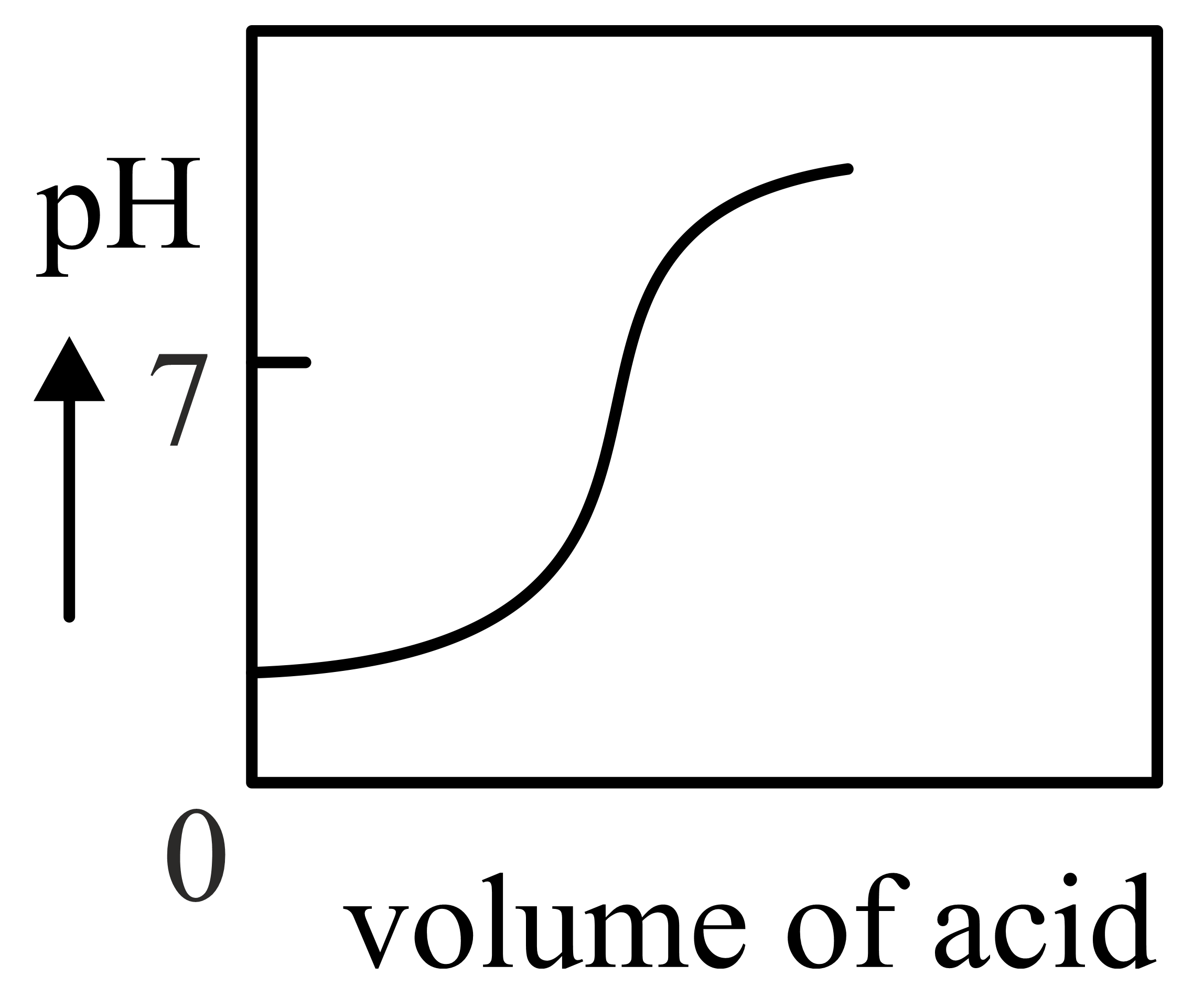
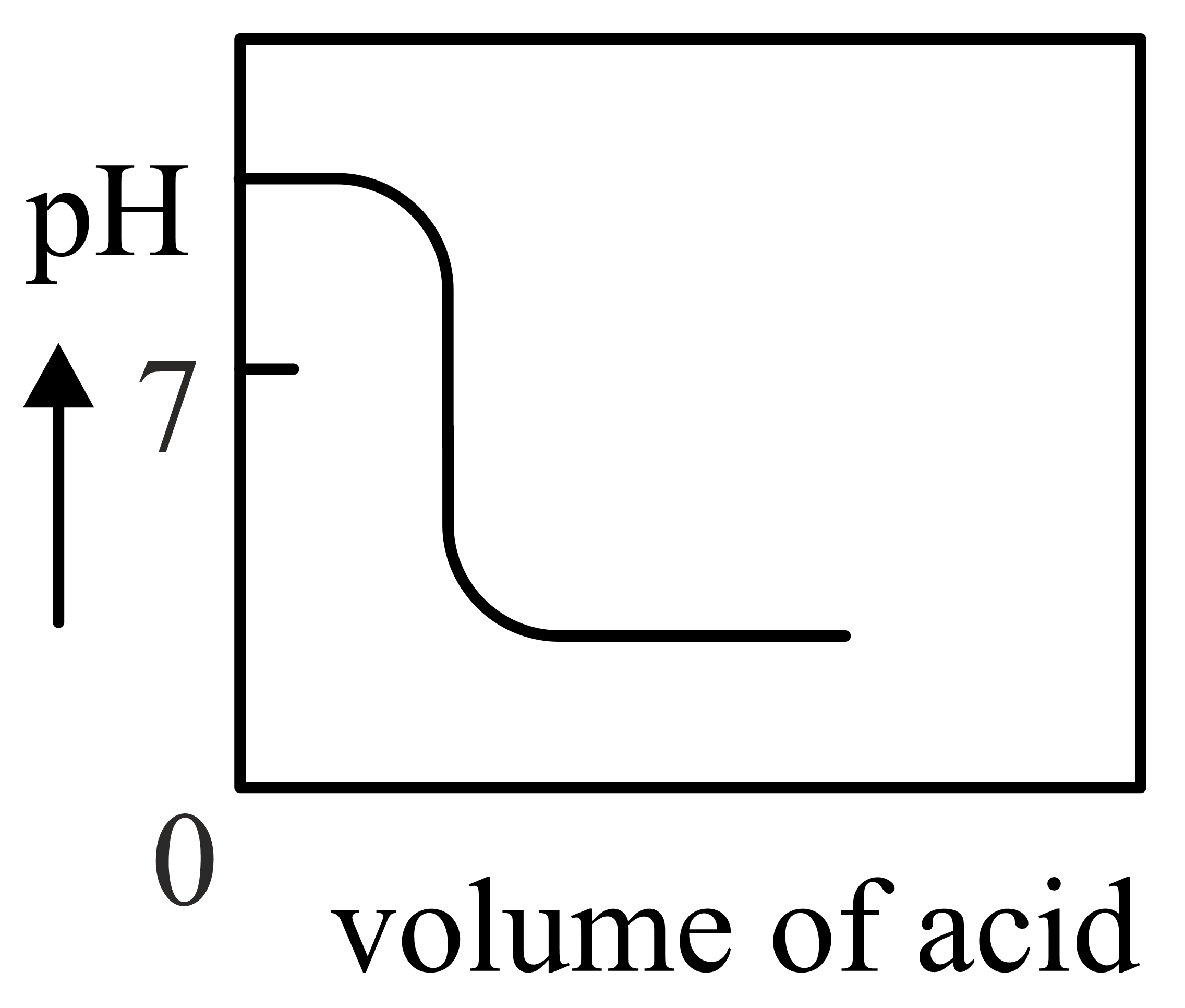
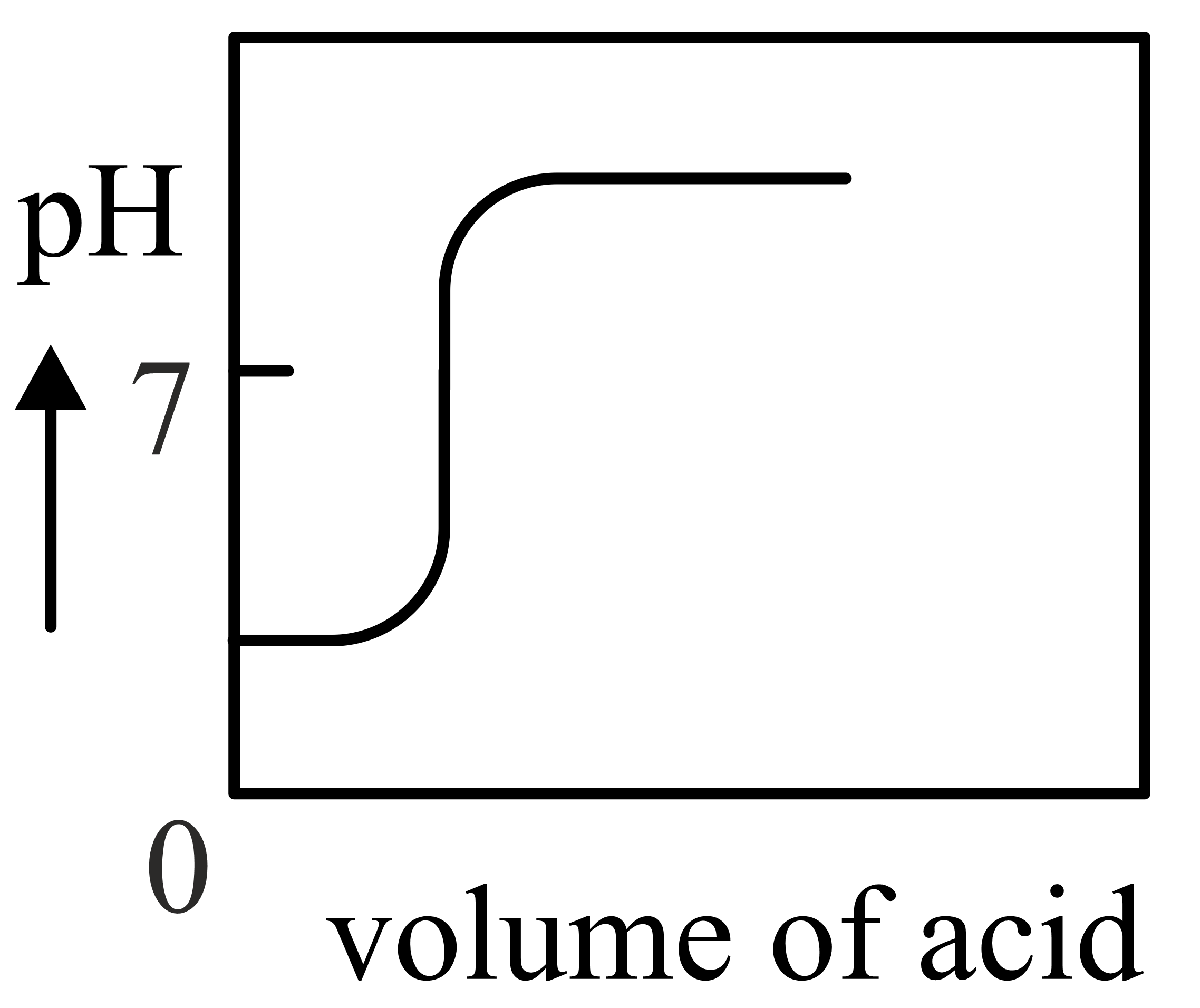
Important Questions on Redox Reactions and Electrochemistry
In the following passage there are blanks, each of which has been numbered. There are questions given below the passage and for each question, four words are suggested, one of which fits the blank appropriately. Find out the appropriate word in each case.
Whenever I go into a bank, I feel scared. Everybody and everything that I see there (1) ____ me. As for the manager the sight (2) ____ him simply terrifies me and (3) ____ me want to runaway (4) _____ I can. As soon as I (5) ____ the door of the bank I lose my head (6) ____ when I try to do any (7) ____ there, I behave like an idiot. I cannot explain (8) ____ for this but that is how it (9) ____ has been that is how it is (10) ____.
Select the most appropriate option for blank No. 10.
Given below are two statements: one is labelled as Assertion (A) and the other is labelled as Reason (R).
Assertion (A) : An aqueous solution of when for volumetric analysis, its concentration should be checked before the use.
Reason (R) : On aging, solution absorbs atmospheric .
In the light of the above statements, choose the correct answer from the options given below.
(Unbalanced)
Statement I : In the titration between strong acid and weak base methyl orange is suitable as an indicator.
Statement II : For titration of acetic acid with $\mathrm{NaOH}$ phenolphthalein is not a suitable indicator.
In the light of the above statements, choose the most appropriate answer from the options given below:
of hypo solution is used for the titration of of copper sulphate solution, in the presence of excess of using starch as an indicator. The molarity of is found to be (nearest integer)
Given :
Which of the following statements is correct ?
Find the nonnality of solution, if of it is required to react completely with of solution. ( Molar mass of )

