EASY
Earn 100
The SI unit for heat capacity is joule per kelvin.
(a)True
(b)False
50% studentsanswered this correctly
Important Questions on Thermal Properties of Matter
MEDIUM
Two different liquids of same mass are kept in two identical vessels, which are placed in a freezer that extracts heat from them at the same rate causing each liquid to transform into a solid. The schematic figure below shows the temperature T vs time t plot for the two materials. We denote the specific heat of materials in the liquid (solid) states to be and respectively.
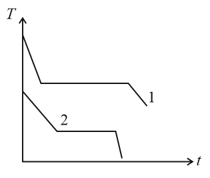
HARD
HARD
The figure below shows the variation of specific heat capacity () of a solid as a function of temperature (). The temperature is increased continuously from to at a constant rate. Ignoring any volume change, the following statement (s) is (are) correct to a reasonable approximation.
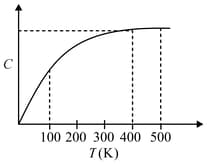
MEDIUM
EASY
(Take gas constant )
HARD
EASY
MEDIUM
MEDIUM
EASY
EASY
MEDIUM
HARD
The value of (in to the nearest integer) is___________
HARD
HARD
MEDIUM
MEDIUM
[Take specific heat of water and latent heat of steam
MEDIUM
(Given: room temperature , specific heat of copper )
MEDIUM
HARD
(Specific heat of water is and the density of water is )

