EASY
Earn 100
The SN2 reaction involves back-side attack and therefore results in a "Walden Inversion." For which one of the substrates shown would you be able to demonstrate that such back-side attack with "Walden Inversion" has in fact occurred?
(a)-bromopentane
(b)-bromopentane
(c)-bromopentane
(d)Methyl bromide
50% studentsanswered this correctly
Important Questions on Haloalkanes and Haloarenes
MEDIUM
Which compound is optically active?
HARD
The correct statement about the following chemical reaction is
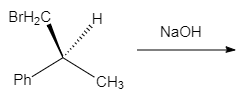
HARD
Which of the following compound is optically inactive?
MEDIUM
The major product of the following reaction is:
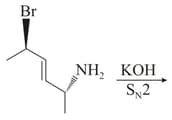
EASY
Two possible stereo-structures of , which are optically active, are called:
EASY
The incorrect statement regarding chirality is :
MEDIUM
How many chiral compounds are possible on monochlorination of -methylbutane?
MEDIUM
Predict the stereochemistry of the product formed if an optically active alkyl halide undergoes substitution reaction by mechanism.
EASY
The number of chiral carbons present in the molecule given below is..............
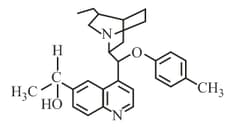
HARD
The number of chiral carbon present in peptide, is:
MEDIUM
Which of the following compound is optically inactive?
EASY
Which of the following structures represents a chiral compound?
MEDIUM
Why dextro and laevo – rotatory isomers of Butan-2-ol are difficult to separate by fractional distillation ?
MEDIUM
The major product of the following reaction is
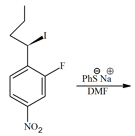
EASY
Identify the chiral molecule in the following pair:
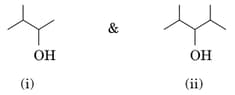
EASY
Give reason:
Racemic mixture is optically inactive.
EASY
Which of the following has a chiral
EASY
The number of chiral carbons in chloramphenicol is ____________.
MEDIUM
Which of the following compounds will show retention in configuration on nucleophile substitution by ?

