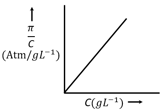The Total pressure observed by mixing two liquid is when their mole fractions are respectively. The Total pressure becomes if the mole fractions are changed to respectively for . The vapour pressure of pure is ______ . (Nearest integer) Consider the liquids and solutions behave ideally

Important Questions on Solutions
The osmotic pressure of solutions of PVC in cyclohexanone at are plotted on the graph. The molar mass of PVC is (Nearest integer)
(Given : )
The number of pairs of the solution having the same value of the osmotic pressure from the following is
(Assume ionization)
A. and
B. and
C. (aq) and
D. and
E. and
Solid Lead nitrate is dissolved in litre of water. The solution was found to boil at . When of is added to the resulting solution, it was observed that the solution froze at . The solutbility product of formed is _____ at . (Nearest integer)
Given : and . Assume molality to be equal to molarity in all cases.
Match List-I and List-II
| List-I | List-II | ||
| A. | Osmosis | I. | Solvent molecules pass through semi permeable membrane towards solvent side. |
| B. | Reverse osmosis | II. | Movement of charged colloidal particles under the influence of applied electric potential towards oppositely charged electrodes |
| C. | Electro osmosis | III. | Solvent molecules pass through semi permeable membrane towards solution side |
| D. | Electrophoresis | IV. | Dispersion medium moves in an electric field. |
Choose the correct answer from the options given below:
Match List I with List II.
| List I | List II | ||
| A. | van't Hoff factor, i |
I. | Cryoscopic constant |
| B. | II. | Isotonic solutions | |
| C. | Solutions with same osmotic pressure | III. | |
| D. | Azeotropes | IV. | Solutions with same composition of vapour above it |
Choose the correct answer from the options given below:
A bottle of soft drink has dissolved in it. Assuming behaves as an ideal gas, the volume of the dissolved at STP is _____ . (Nearest integer)
Given: At STP, molar volume of an ideal gas is
A solution containing of a non-volatile solute in of water boils at . The molecular mass of the solute is _____ . (Nearest integer)
Given, water boils at for water
Lead storage battery contains by weight solution of . The van't Hoff factor is at this concentration. The temperature in Kelvin at which the solution in the battery will freeze is _____ (Nearest integer).
Given