EASY
Earn 100
The adiabatic efficiency is given by
(a)
(b)
(c)
(d)
50% studentsanswered this correctly
Important Questions on Heat and Thermodynamics
MEDIUM
HARD
(Given is gas constant)
HARD
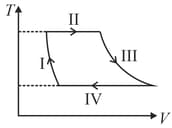
| Column – 1 | Column – 2 | Column – 3 |
| (I) | (i) Isothermal | (P) 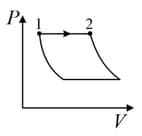 |
| (II) | (ii) Isochoric | (Q) 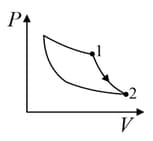 |
| (III) | (iii) Isobaric | (R) 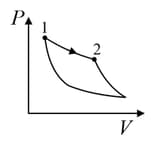 |
| (IV) | (iv) Adiabatic | (S) 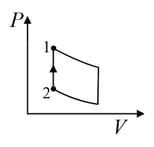 |
HARD
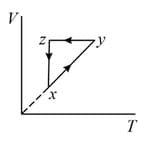
The - diagram that best describes this cycle is: (Diagrams are schematic and not to scale)
MEDIUM
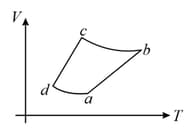
The corresponding P - V diagram for the process is (all figures are schematic and not drawn to scale) :
EASY
HARD
[ is the gas constant]
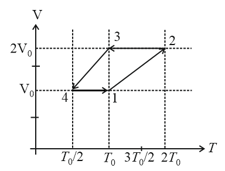
EASY
HARD
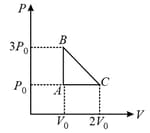
MEDIUM
MEDIUM
HARD
EASY
EASY
EASY
EASY
EASY
EASY
EASY
For the given cyclic process as shown for a gas, the work done is:
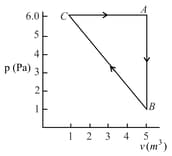
HARD