The adjoining figure show the plot of an ideal gas taken through a cycle The part is a semi-circle and is half of an ellipse. Then select the correct statement from the following :
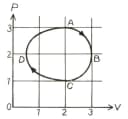

the process during the path is isothermal

Important Questions on Internal Energy : First Law of Thermodynamics : Specific Heat
of water is heated from to. Ignoring the slight expansion of the water, the change in its internal energy is specific heat of water is .
A thermally insulated vessel contains an ideal gas of molecular mass and a ratio of specific heats . It is moving with speed and is suddenly brought to rest. Assuming no heat is lost to the surroundings, its temperature increases by,
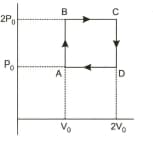
Helium gas goes through a cycle consisting of two isochoric and two isobaric lines as shown in the figure. Efficiency of this cycle is nearly : Assume the gas to be close to ideal gas
In a process carried on an ideal gas, Then, of the gas:
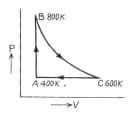
One mole of diatomic ideal gas undergoes a cyclic process as shown in figure. The process is adiabatic. The temperature at , and are , and respectively. Choose the correct statement.
If denote respectively the heat added, change in internal energy and the work done in a closed cycle process, then:
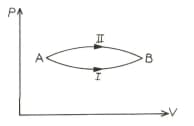
A system goes from the state to the state via two process as shown figure. If are the changes in internal energies in the process respectively, then
An ideal is filled in a closed, rigid and thermally-insulated container. A coil of resistance and carrying a current of supplies heaT to the gas. The change in the internal energy of the gas after minutes will be:
