EASY
Earn 100
The atomic symbols of some elements are given below:
. Which of the elements from the list belongs to period 4?
64.29% studentsanswered this correctly
Important Questions on Classification of Elements and Periodicity in Properties
MEDIUM
In which of the following options, the law of triad is applicable?
EASY
Mendeleev's periodic law states that the properties of elements are a periodic function of their
MEDIUM
Which one of the following statements for D.I. Mendeleev, is incorrect ?
EASY
Identify the incorrect match.
| Name IUPAC | Official Name |
| (a) Unnilunium | (i) Mendelevium |
| (b) Unniltrium | (ii) Lawrencium |
| (c) Unnilhexium | (iii) Seaborgium |
| (d) Unununium | (iv) Darmstadtium |
MEDIUM
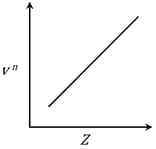
It is observed that characteristic X-ray spectra of elements show regularity. When frequency to the power 'n' i.e. of X-rays emitted is plotted against atomic number , the following graph is obtained.
The value of 'n' is
EASY
The atomic number of the element unnilennium is :
EASY
Which one of the following element is represented as Eka-Silicon in Mendeleev's periodic table?
EASY
The element has been discovered recently. It will belong to which of the following family/group and electronic configuration?
EASY
Match List - I with List - II
| LIST-I (Atomic number) |
LIST-II (Block of periodic table) |
||
| (A) | I. | -block | |
| (B) | II. | -block | |
| (C) | III. | -block | |
| (D) | IV. | -block | |
Choose the correct answer from the options given below:
MEDIUM
The pair of lanthanides with exceptionally high ionisation enthalpy than neighbouring elements:
MEDIUM
Which of the given atoms has the greatest electron affinity?
MEDIUM
The electronic configurations of bivalent europium and trivalent cerium are:
(atomic number: , ,
MEDIUM
The neutral oxide among the following is
EASY
Which of the following is a metalloid?
EASY
The electron of an element with an atomic number of enters the orbital:
EASY
For the following Assertion and Reason, the correct option is:
Assertion: For hydrogenation reactions, the catalytic activity increases from Group to Group metals with maximum activity shown by Group elements.
Reason: The reactants are most strongly adsorbed on group elements.
EASY
The IUPAC symbol for the element with atomic number would be:
MEDIUM
Henry Moseley studied characteristic -ray spectra of elements. The graph which represents his observation correctly is
Given Frequency of X-ray emitted
Atomic number
EASY
The last element of the - block in period is represented by the outermost electronic configuration:
EASY
The electronic configurations of Eu (Atomic no. ), Gd (Atomic no. ) and Tb (Atomic no. ) are: