The boiling points of argon, nitrogen and oxygen are and respectively. Which of these gases will distill first from their mixture in the air? Justify your answer.
Important Questions on Matter: Elements, Compounds and Mixtures
Which of the following are NOT correct methods for separating the components of given mixtures?
I. The mixture of Iodine and sodium chloride by sublimation.
II. Plant pigments by chromatography.
III. Mixture of acetic acid and water by separating funnel.
IV. Oxygen, argon and nitrogen from air by fractional distillation.
Given below are two statements:
Statement-I: Retardation factor can be measured in meter/centimetre.
Statement-II: value of a compound remains constant in all solvents.
Choose the most appropriate answer from the options given below:
Using the provided information in the following paper chromatogram:
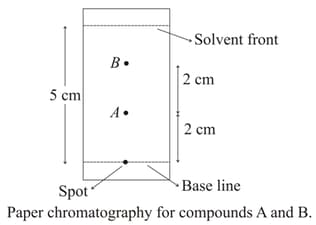
The calculated value of .
At a refinery, petroleum is separated into several components by a process called fractional distillation using a fractionating column. Which of the following statement is incorrect about the process?
(I) Temperature decreases from the bottom to the top of the column
(II) At each level in the column only one compound is collected
(III) The fraction collecting at the top of the column is less volatile
(IV) The fraction with the highest boiling point condenses first and gets collected near the base of the fractionating column.
CASE: Separating the components of seawater
Student A is given the task of designing an experiment to separate and collect the components of a sample of seawater, (aq). They are provided with standard laboratory apparatus. Following the experiment, the student recorded the following data for analysis.
The initial volume of seawater
The volume of water recovered
Mass of salt recovered
The student in their research for a laboratory report discovered the following facts.
| Sodium chloride | Water | |
| Molar mass | ||
| Solubility in water | - | |
| Boiling point | ||
| Appearance | White crystalline substance | Colourless liquid |
All the water could not be collected during the separation process. Which of the following is not a reason for this?
Distilled water cannot be used for which among the following purposes?
CASE: Separating the components of seawater
Student A is given the task of designing an experiment to separate and collect the components of a sample of seawater, (aq). They are provided with standard laboratory apparatus. Following the experiment, the student recorded the following data for analysis.
The initial volume of seawater
The volume of water recovered
Mass of salt recovered
The student in their research for a laboratory report discovered the following facts.
| Sodium chloride | Water | |
| Molar mass | ||
| Solubility in water | - | |
| Boiling point | ||
| Appearance | White crystalline substance | Colourless liquid |
How can one obtain the water from the seawater?
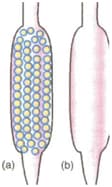
Which of the tubes in (a) and (b) will be more effective as a condenser in the distillation apparatus?
Ink is soluble in water. How can you get pure water from a mixture of ink and water?

