The boiling points of the hydrogen halides are shown in the table.
Hydrogen halide
HF
HCl
HBr
HI
Boiling point / °C
+20
–85
–67
–35
Explain the trend in boiling points from to .

Important Questions on Chemical Bonding
The boiling points of the hydrogen halides are shown in the table.
| Hydrogen halide | HF | HCl | HBr | HI |
| Boiling point / °C | +20 | –85 | –67 | –35 |
Explain why the boiling point of HF is so much higher than the boiling point of HCl.
The table lists the boiling points of some Group 15 hydrides.
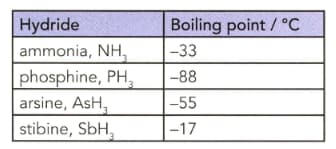
Explain why the boiling point of ammonia does not follow the trend in the boiling points from phosphine to stibine.
Explain the following difference in terms of the type of bonding present.
Aluminium oxide has a melting point of 2980 °C but aluminium chloride changes to a vapour at 178 °C.
Explain the following difference in terms of the type of bonding present.
Magnesium chloride conducts electricity when molten but not when solid.
Explain the following difference in terms of the type of bonding present.
Iron conducts electricity when solid but the ionic solid iron(II) chloride does not conduct when solid.
Explain the following difference in terms of the type of bonding present.
Sodium sulfate dissolves in water, but sulfur does not.
Explain the following difference in terms of the type of bonding present:
Propanol, , is soluble in water but propane , is not.
Explain the following difference in terms of the type of bonding present:
A solution of hydrogen chloride in water conducts electricity.
