EASY
Earn 100
The bond order of the N−O bonds in ion is
(a)1.33
(b)1.50
(c)1.00
(d)0.33
50% studentsanswered this correctly
Important Questions on Chemical Bonding and Molecular Structure
MEDIUM
MEDIUM
EASY
How many ions of the following have a bond order of
MEDIUM
MEDIUM
MEDIUM
The bond lengths (in of and are:
MEDIUM
EASY
EASY
MEDIUM
MEDIUM
EASY
MEDIUM
EASY
MEDIUM
EASY
EASY
EASY
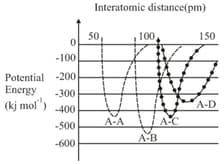
HARD
MEDIUM

