EASY
12th ICSE
IMPORTANT
Earn 100
The cell reaction is spontaneous or feasible when emf of the cell is:
(a)negative
(b)positive
(c)zero
(d)either positive or negative
50% studentsanswered this correctly

Important Questions on Electrochemistry
MEDIUM
12th ICSE
IMPORTANT
Calculate the mass of silver deposited at cathode when a current of amperes is passed through a solution of for minutes. (atomic mass: ).
MEDIUM
12th ICSE
IMPORTANT
Calculate the emf and for the cell reaction at ·
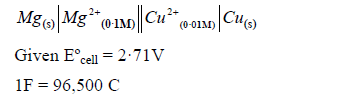
EASY
12th ICSE
IMPORTANT
EASY
12th ICSE
IMPORTANT
MEDIUM
12th ICSE
IMPORTANT
The resistance of a conductivity cell containing solution at is . What is the cell constant and molar conductivity of solution, if the conductivity of this solution is at ?
EASY
12th ICSE
IMPORTANT
MEDIUM
12th ICSE
IMPORTANT
Calculate the emf and for the given cell at :
Given: .
MEDIUM
12th ICSE
IMPORTANT
Calculate the degree of dissociation of acetic acid, if its molar conductivity is .
Given: and .
