MEDIUM
AS and A Level
IMPORTANT
Earn 100
The chloride of an unknown element, X, is a liquid at . This chloride reacts with water, giving off white fumes and leaving an acidic solution.
i) Does element X belong to Group 1, Group 2 or Group 15 of the Periodic Table?
ii) What type of reaction takes place between X and water?
iii) Identify the white fumes given off when X reacts with water.

Important Questions on Periodicity
MEDIUM
AS and A Level
IMPORTANT
The chloride of an unknown element Y is a solid at . This chloride does not react with water but dissolves to give a neutral solution. Does element Y belong to Group 1, Group 14 or Group 16 of the Periodic Table?
EASY
AS and A Level
IMPORTANT
Explain what is meant by the term 'periodic property'.
MEDIUM
AS and A Level
IMPORTANT
The graph shows how a periodic property varies when plotted against atomic number for Period 3 (sodium to argon).
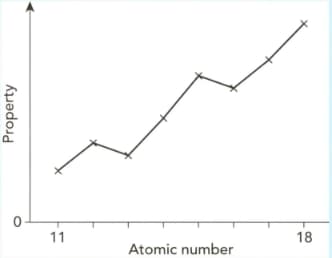
(i) Identify the property.
(ii) Explain the overall trend across the period.
MEDIUM
AS and A Level
IMPORTANT
i) Describe how the atomic radius varies across Periods 2 and 3.
ii) Explain this trend.
EASY
AS and A Level
IMPORTANT
(i) Describe how the atomic radius varies down each group of the periodic table.
(ii) Explain this trend.
MEDIUM
AS and A Level
IMPORTANT
Describe the acid-base nature of the solutions obtained when sodium chloride is added to water. Use equations to illustrate your answer.
MEDIUM
AS and A Level
IMPORTANT
Describe the acid-base nature of the solutions obtained when sulphur trioxide is added to water. Use equations to illustrate your answer.
MEDIUM
AS and A Level
IMPORTANT
Describe the acid-base nature of the solutions obtained when sodium oxide is added to water. Use equations to illustrate your answer.
