EASY
Earn 100
The colour of calcium nitrate salt is dirty yellow.
(a)True
(b)False
75% studentsanswered this correctly
Important Questions on Qualitative Analysis (OoS)
EASY
HARD
EASY
Match List-I with List-II and select the correct answer using the codes given below.
| List I | List II | ||
| A. | Rinmann's green | 1. | |
| B. | Verdigris | 2. | |
| C. | Corrosive sublimate | 3. | |
| D. | Lithopone | 4. |
MEDIUM
MEDIUM
HARD
HARD
HARD
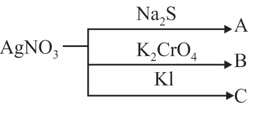
The colour of ppt. and respectively is
EASY
HARD
EASY
EASY
MEDIUM
EASY
EASY
HARD
EASY
MEDIUM
Identity the salt from the observation given below:
When dilute is added to a salt , a brisk effervescence is produced and the gas turns lime water milky. When solution is added to the above mixture (after adding dilute ), it produces a white precipitate which is soluble in excess solution.
MEDIUM
MEDIUM

