HARD
JEE Advanced
IMPORTANT
Earn 100
The complex is diamagnetic, but is paramagnetic and has two unpaired electrons. Explain these observations and deduce the structures of the two complexes.

Important Questions on Coordination Compounds
MEDIUM
JEE Advanced
IMPORTANT
What methods could be used to distinguish between cis and trans isomers of a complex?
HARD
JEE Advanced
IMPORTANT
Name the individual isomers of each of the following:
(a)
(b)
(c)
(d)
(e) 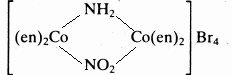
(f)
(g)
HARD
JEE Advanced
IMPORTANT
Account for the following:
(a) is tetrahedral
(b) is square planar
(c) is octahedral
MEDIUM
JEE Advanced
IMPORTANT
MEDIUM
JEE Advanced
IMPORTANT
MEDIUM
JEE Advanced
IMPORTANT
MEDIUM
JEE Advanced
IMPORTANT
Write the formula for the following complex:
nickel hexachloroplatinate
MEDIUM
JEE Advanced
IMPORTANT
Write the formula for the following complex:
tetraamminecopper sulphate
