MEDIUM
MHT-CET
IMPORTANT
Earn 100
The compound '', i.e., butyronitrile is subjected to the Stephen reaction by using and dilute , followed by the acid hydrolysis to give compound '', which is having a buttery odour, and it is used in margarine. The addition of hydrogen cyanide to the compound '', in the presence of a small amount of base, gives compound ''. Identify the compounds '' and ''.
(a)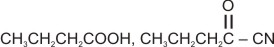
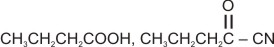
(b)

(c)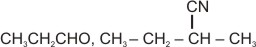

(d)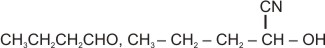
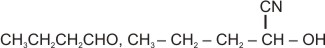
50% studentsanswered this correctly

Important Questions on Organic Reactions
EASY
MHT-CET
IMPORTANT
The compound i.e., propanamide is treated with bromine and alcoholic sodium hydroxide to give compound . The acid catalysed addition of valeraldehyde to compound yields compound which is well known as a 'Schiff base'. What are the structures of compounds and ?
MEDIUM
MHT-CET
IMPORTANT
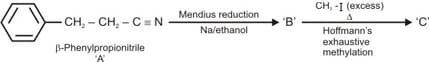
Identify the products and .
HARD
MHT-CET
IMPORTANT
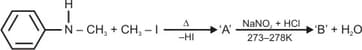
Predict the products in the following reaction:
MEDIUM
MHT-CET
IMPORTANT
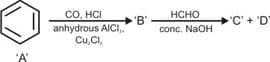
Predict the products in the following reactions:
EASY
MHT-CET
IMPORTANT
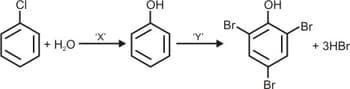
Identify '' and '' in the following example.
MEDIUM
MHT-CET
IMPORTANT
Bromobutane is treated with in the presence of dry acetone to give compound . The compound is boiled with moist silver oxide to give compound . Identify the compounds and . How many optical isomers are possible for compound ?
MEDIUM
MHT-CET
IMPORTANT
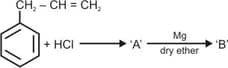
Identify and in the following reaction:
MEDIUM
MHT-CET
IMPORTANT
The compounds and are the structural isomers of each other with the molecular formula . Compound is a linear chain and optically inactive while is optically active. When compound is treated with aqueous solution, it gives compound whereas compound when treated with aqueous solution, it gives compound . Identify compounds and .
