EASY
Upper Secondary: IGCSE
IMPORTANT
Earn 100
The compound zinc sulphide has a structure like this:
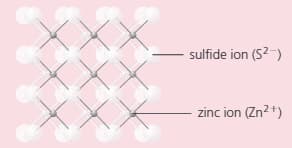
Which type of bonding does zinc sulphide have?


Important Questions on Atoms Combining
MEDIUM
Upper Secondary: IGCSE
IMPORTANT
- Cotton being woven to make sheets.
Is it a chemical change or a physical change? Give reason.
MEDIUM
Upper Secondary: IGCSE
IMPORTANT
MEDIUM
Upper Secondary: IGCSE
IMPORTANT
EASY
Upper Secondary: IGCSE
IMPORTANT
EASY
Upper Secondary: IGCSE
IMPORTANT
Most transition elements can form more than one type of ion.
| Ion | Name | Example of compound |
| Copper ion | Copper oxide, | |
| Copper ion | Copper oxide, | |
| Iron ion | Iron chloride, | |
| Iron ion | Iron chloride, |
The in the name tells you that the ion has a charge of . What do the and show?
EASY
Upper Secondary: IGCSE
IMPORTANT
The compound zinc sulphide has a structure like this:
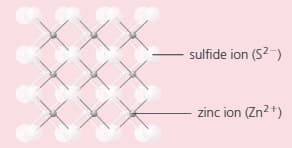
Look carefully at the structure. How many:
i) sulphur ions are joined to each zinc ion?
ii) zinc ions are joined to each sulphur ion?
MEDIUM
Upper Secondary: IGCSE
IMPORTANT
The compound zinc sulphide has a structure like this:
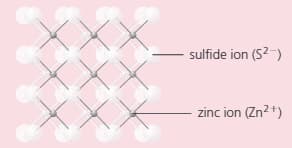
i) Deduce the formula of zinc sulphide.
ii) Is this formula consistent with the charges on the two ions? Explain your answer.
EASY
Upper Secondary: IGCSE
IMPORTANT
The properties of metals can be explained by the structure and bonding within the metal lattice. Describe the bonding in metals.
