MEDIUM
JEE Main/Advance
IMPORTANT
Earn 100
The conductometric titration curve (of conductance vs of obtained when acetic acid is titrated against is:
(a)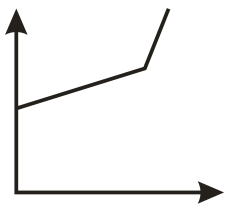

(b)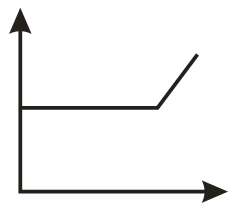

(c)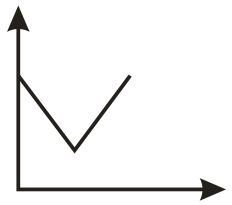

(d)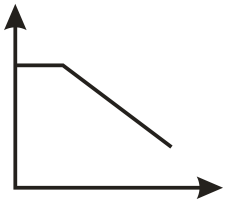

50% studentsanswered this correctly

Important Questions on Electrochemistry
MEDIUM
JEE Main/Advance
IMPORTANT
In an alkaline energy cell the overall cell reaction is as follows:
Which of the following reactions is taking place at the cathode?
EASY
JEE Main/Advance
IMPORTANT
MEDIUM
JEE Main/Advance
IMPORTANT
HARD
JEE Main/Advance
IMPORTANT
MEDIUM
JEE Main/Advance
IMPORTANT
at . What is the value of ?
MEDIUM
JEE Main/Advance
IMPORTANT
HARD
JEE Main/Advance
IMPORTANT
Nickel metal is added to a solution containing and . Use the standard reduction potential to determine which of the following reaction (s) will occur.
Reaction :
Reaction :
Reactions:
MEDIUM
JEE Main/Advance
IMPORTANT
An electrochemical cell constructed for the reaction :
has an . The standard reduction potential for is . What is the standard reduction potential for ?
