MEDIUM
Earn 100
The correct molecular orbital diagram for molecule in the ground state is
(a)

(b)
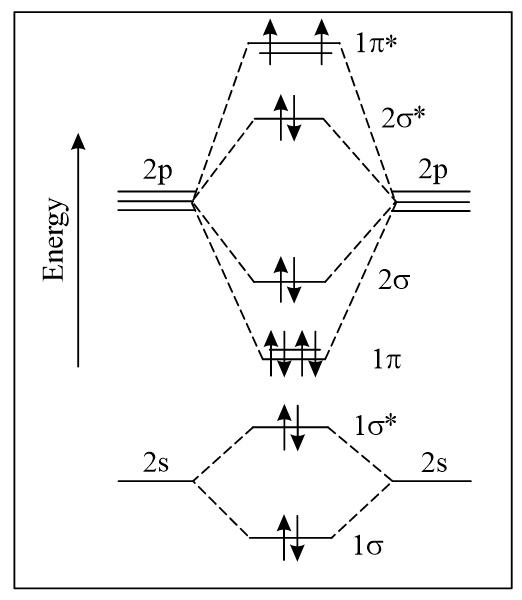
(c)
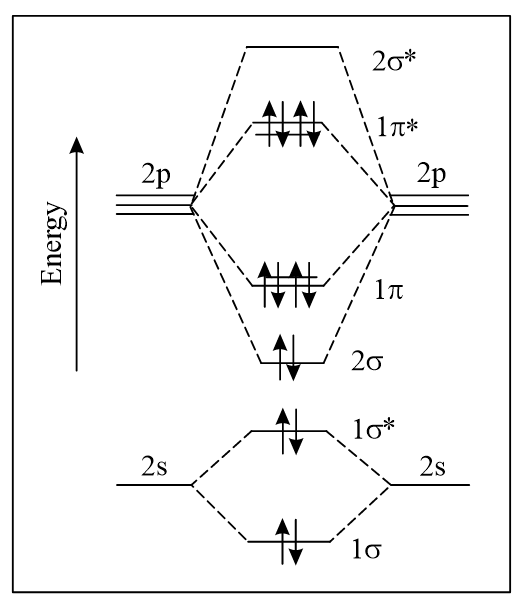
(d)
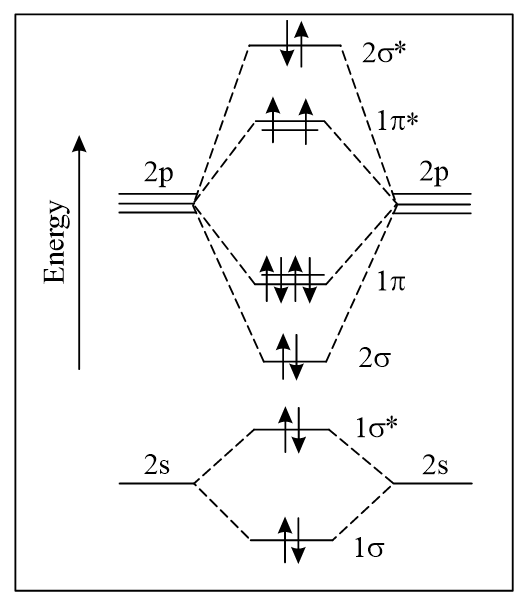
66.67% studentsanswered this correctly
Important Questions on Chemical Bonding
EASY
HARD
Decreasing order of stability of and is:
EASY
HARD
EASY
MEDIUM
EASY
HARD
EASY
HARD
MEDIUM
MEDIUM
MEDIUM
EASY
MEDIUM
HARD
Among the following molecules/ ions,
Which one is diamagnetic and has the shortest bond length?
MEDIUM
MEDIUM
HARD
(superoxide), dimeric sulphur in vapour phase,
MEDIUM

