The correct order of boiling points of noble gases is
Important Questions on Elements of Groups 16, 17 and 18
| Column I | Column II | ||
| (a) | (i) | Distorted octahedral | |
| (b) | (ii) | Square planar | |
| (c) | (iii) | Pyramidal | |
| (d) | (iv) | Square pyramidal |
Account for the following:
Boiling point of noble gases increases from to .
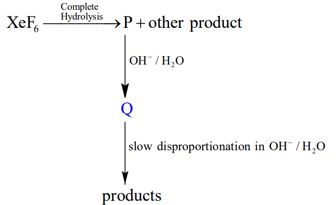
Under ambient conditions, the total number of gases released as products in the final step of the reaction scheme shown below is
Among the following molecules,
(i)
(ii)
(iii)
those having same number of lone pairs on are:
Answer the following :
Noble gases have very low boiling points. Why?
Given below are two statements: One is labelled as Assertion A and the other is labelled as Reason R.
Assertion A: Amongst and ; of activated charcoal adsorbs more of .
Reason R: The critical volume and critical pressure (atm) is highest for Krypton but the compressibility factor at critical point is lowest for Krypton.
In the light of the above statements, choose the correct answer from the options given below.
Noble gases are named because of their inertness towards reactivity. Identify an incorrect statement about them.

