MEDIUM
NEET
IMPORTANT
Earn 100
The correct order of dipole moment is
(a)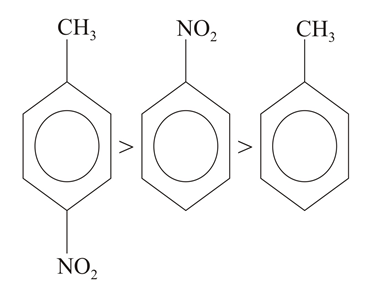

(b)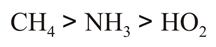
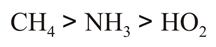
(c)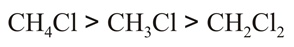
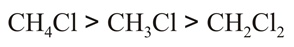
(d)All of these
50% studentsanswered this correctly

Important Questions on Chemical Bonding and Molecular Structure
EASY
NEET
IMPORTANT
HARD
NEET
IMPORTANT
MEDIUM
NEET
IMPORTANT
MEDIUM
NEET
IMPORTANT
Consider the two molecules given below:
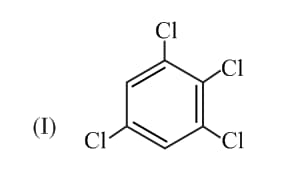
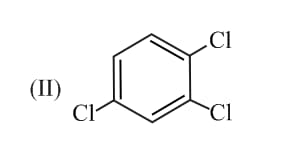
If the dipole moment of bond is , the ratio of the dipole moment of and is:
MEDIUM
NEET
IMPORTANT
MEDIUM
NEET
IMPORTANT
HARD
NEET
IMPORTANT
EASY
NEET
IMPORTANT
