MEDIUM
NEET
IMPORTANT
Earn 100
The correct order of dipole moments for the following molecules is
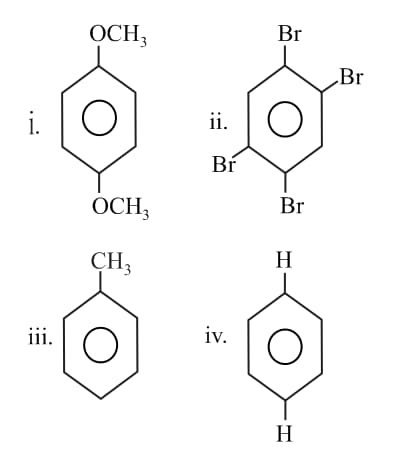

(a)
(b)
(c)
(d)
50% studentsanswered this correctly

Important Questions on Chemical Bonding and Molecular Structure
MEDIUM
NEET
IMPORTANT
Identify the correct match with respect to the dipole moment of given molecules.
MEDIUM
NEET
IMPORTANT
Identify the incorrect statement for molecule.
MEDIUM
NEET
IMPORTANT
In which of the following, the number of orbital dipoles is equal to the number of the bond moments?
HARD
NEET
IMPORTANT
A molecule has equal dipole moment as that of toluene. Then, is:
EASY
NEET
IMPORTANT
For the given molecule which is a linear molecule, the dipole moment of bond is . If bond distance is , then magnitude of charge on central atom is:
MEDIUM
NEET
IMPORTANT
Which one of the following molecules has a non-zero dipole moment but is planar?
MEDIUM
NEET
IMPORTANT
Which of the following orders incorrectly represents the property indicated?
MEDIUM
NEET
IMPORTANT
The bond with the maximum covalent character is:
