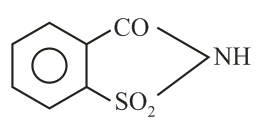
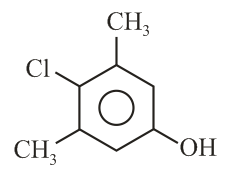
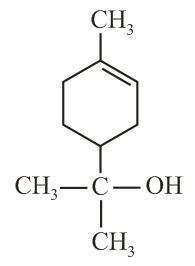
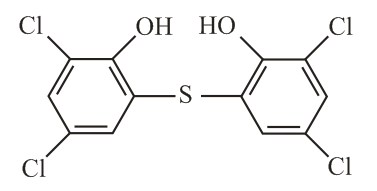
The correct structure of Bithional is

Important Points to Remember in Chapter -1 - Chemistry in Everyday Life from Embibe Experts Gamma Question Bank for Engineering Chemistry Solutions
1. Drugs:
(i) Drugs are low molecular mass substances which interact with targets in the body and produce a biological response.
(ii) Main role of drugs is to either increase or decrease the role of enzyme catalysed reactions.
(iii) Inhibition of enzymes is a common role of drug action.
2. Medicines:
(i) Medicines are chemicals that are useful in the diagnosis, prevention, and treatment of diseases.
3. Therapeutic effect:
(i) Desirable or beneficial effects of a drug like the treatment of symptoms and cure of a disease on a living body is known as therapeutic effect.
4. Functions of enzymes:
(i) The first function of an enzyme is to hold the substrate for a chemical reaction. Active sites of enzymes hold the substrate molecule in a suitable position so that it can be attacked by the reagent effectively.
(ii) The second function of an enzyme is to provide functional groups that will attack the substrate and carry out chemical reactions.
(iii) Enzyme inhibitor is a drug which inhibits catalytic activity of enzymes or blocks the binding site of the enzyme and eventually prevents the binding of substrate with enzyme.
5. Drug can inhibit attachment of substrate on active site of enzymes in following ways:
(i) Competitive Inhibitors are the drugs that compete with the natural substrate for their attachment on the active sites of enzymes.
(ii) Non-Competitive Inhibition:
(a) Some drugs do not bind to the enzyme's active site, instead bind to a different site of enzyme called allosteric site.
(b) This binding of inhibitors at allosteric sites changes the shape of the active site in such a way that substrate cannot recognise it.
(c) If the bond formed between an enzyme and an inhibitor is a strong covalent bond and cannot be broken easily, then the enzyme is blocked permanently.
(d) The body then degrades the enzyme-inhibitor complex and synthesizes the new enzyme.
(iii) Receptors:
(a) Proteins which are vital for the communication system in the body are called receptors.
(b) Receptors show selectivity for one chemical messenger over the other because their binding sites have different shape, structure, and amino acid composition.
(iv) Receptors as Drug Targets:
(a) In the body, the message between two neurons and that between neurons to muscles is communicated through chemical messengers.
(b) They are received at the binding sites of receptor proteins.
(c) To accommodate a messenger, the shape of the receptor site changes which brings about the transfer of message into the cell.
(d) Chemical messenger gives a message to the cell without entering the cell.
6. Antagonists and Agonists:
(i) Drugs that bind to the receptor site and inhibit its natural function are called antagonists.
(ii) These are useful when blocking messages are required.
(iii) Drugs that mimic the natural messenger by switching on the receptor are called agonists.
(iv) These are useful when there is a lack of natural chemical messengers.
7. Therapeutic action of different classes of drugs:
(i) Antacid:
(a) Chemical substances which neutralize excess acid in the gastric juices and give relief from acid indigestion, acidity, heart burns and gastric ulcers.
(ii) Antihistamines:
(a) Chemical substances which diminish or abolish the effects of histamine released in the body and hence prevent allergic reactions. Examples: Brompheniramine (Dimetapp) and terfenadine (Seldane).
(iii) Neurologically Active Drugs:
(a) Drugs which have a neurological effect i.e., affect the message transfer mechanism from nerve to receptor.
(iv) Tranquilizers:
(a) Chemical substances used for the treatment of stress and mild or severe mental diseases.
(b) Examples: Derivatives of barbituric acids like veronal, amytal, Nembutal, luminal, seconal.
(v) Analgesics:
(a) Chemical substances used to relieve pain without causing any disturbances in the nervous system like impairment of consciousness, mental confusion, in coordination or paralysis etc.
8. Classification of Analgesics:
(i) Non-narcotic analgesics:
(a) They are non-addictive drugs.
(b) Examples: Aspirin, ibuprofen, Naproxen, Diclofenac Sodium.
(ii) Narcotic analgesics:
(a) When administered in medicinal doses, these drugs relieve pain and produce sleep.
(b) Examples: Morphine and its derivatives.
9. Antimicrobials:
(i) Drugs that tend to destroy/prevent development or inhibit the pathogenic action of microbes such as bacteria (antibacterial drugs), fungi (antifungal agents), virus (antiviral agents), or other parasites (anti-parasitic drugs) selectively.
10. Anti-fertility Drugs:
(i) Chemical substances used to prevent conception or fertilization are called antifertility drugs. Examples - Norethindrone, ethynylestradiol (novestrol).
11. Types of antimicrobial drugs:
(i) Antibiotics: Chemical substances produced by microorganisms that kill or prevent the growth of other microbes.
12. Classification of antimicrobial drugs based on the mode of control of microbial diseases:
(i) Bactericidal drugs - Drugs that kill organisms in the body. Examples - Penicillin, Aminoglycosides, Ofloxacin.
(ii) Bacteriostatic drugs - Drugs that inhibit growth of organisms. Examples - Erythromycin, Tetracycline, Chloramphenicol.
13. Classification of antimicrobial drugs based on its spectrum of action:
(i) Broad-spectrum antibiotics - Antibiotics which kill or inhibit a wide range of Gram-positive and Gram-negative bacteria are called broad-spectrum antibiotics. Examples - Ampicillin and Amoxicillin.
(ii) Narrow spectrum antibiotics - Antibiotics which are effective mainly against Gram-positive or Gram-negative bacteria are called narrow-spectrum antibiotics. Examples- Penicillin .
(iii) Limited spectrum antibiotics - Antibiotics effective against a single organism or disease
14. Antiseptics:
(i) Chemical substances that kill or prevent growth of microorganisms and can be applied on living tissues such as cuts, wounds etc, are called antiseptics. Examples - Soframycin, Dettol etc.
15. Disinfectants:
(i) Chemical substances that kill microorganisms but cannot be applied on living tissues such as cuts, wounds etc, are called disinfectants. Examples - Chlorine , bithionol, iodoform etc.
16. Food additives:
(i) Food additives are the substances added to food to preserve its flavour or improve its taste and appearance.
17. Different types of food additives:
(i) Artificial Sweetening Agents: Chemical compounds which give sweetening effect to the food and enhance its flavour, Examples - Aspartame, Sucralose and Alitame.
(ii) Food preservatives: Chemical substances which are added to food material to prevent their spoilage due to microbial growth. Examples - Sugar, Salts, Sodium benzoate
(iii) Food colours: Substances added to food to increase the acceptability and attractiveness of the food product. Examples - Allura Red , Tartrazine
(iv) Nutritional supplements: Substances added to food to improve the nutritional value. Examples -Vitamins, minerals etc.
(v) Fat emulsifiers and stabilizing agents: Substances added to food products to give texture and desired consistency. Examples - Egg yolk (where the main emulsifying chemical is Lecithin)
(vi) Antioxidants: Substances added to food to prevent oxidation of food materials. Examples Butylated HydroxyToluene , Butylated Hydroxy Anisole .
18. Soaps:
(i) It is a sodium or potassium salt of long chain fatty acids like stearic, oleic and palmitic acid.This reaction is known as saponification.
19. Types of soaps:
(i) Toilet soaps are prepared by using better grades of fats and oil sand care is taken to remove excess alkali. Colour and perfumes are added to make these more attractive.
(ii) Transparent soaps are made by dissolving the soap in ethanol and then evaporating the excess solvent.
(iii) In medicated soaps, substances of medicinal value are added. In some soaps, deodorants are added.
(iv) Shaving soaps contain glycerol to prevent rapid drying. A gum called; rosin is added while making them. It forms sodium rosinate which lathers well.
(v) Laundry soaps contain fillers like sodium rosinate, sodium silicate, borax, and sodium carbonate.
(vi) Soaps that float in water are made by beating tiny air bubbles before their hardening.
(vii) Soap chips are made by running a thin sheet of melted soap on to a cool cylinder and scraping off the soaps in small broken pieces.
20. Classification of detergents:
(i) Anionic detergents:
(a) Anionic detergents are sodium salts of sulphonated long chain alcohols or hydrocarbons.
(b) Alkyl hydrogen sulphates formed by treating long chain alcohols with concentrated sulphuric acid are neutralised with alkali to form anionic detergents. Similarly, alkyl benzene sulphonates are obtained by neutralising alkyl benzene sulphonic acids with alkali. Anionic detergents are termed so because a large part of a molecule is an anion.
(c) They are used in household cleaning like dishwasher liquids, laundry liquid detergents, laundry powdered detergents etc.
(d) They are effective in slightly acidic solutions where soaps do not work efficiently.
(ii) Cationic detergents:
(a) Cationic detergents are quaternary ammonium salts of mine with acetates, chlorides or bromides as anions.
(b) Cationic parts possess a long hydrocarbon chain and a positive charge on nitrogen atoms.
(c) Cationic detergents are termed so because a large part of a molecule is a cation. since they possess germicidal properties, they are used as germicides.
(d) They have strong germicidal action but are expensive.
(iii) Non- ionic detergents: T
(a) They do not contain any ion in their constitution.
(b) They are like esters of high molecular mass.
(c) Example: Detergent formed by condensation reaction between stearic acid reacts and polyethylene glycol.
(d) It is used in making liquid washing detergents.
(e) They have effective -bonding groups at one end of the alkyl chain which make them freely water soluble.
(iv) Biodegradable detergents:
(a) Detergents having straight hydrocarbon chains that are easily decomposed by microorganisms. Example: Sodium lauryl sulphate
(v) Non-Biodegradable detergents:
(a) Detergents having branched hydrocarbon chains that are not easily decomposed by microorganisms.
