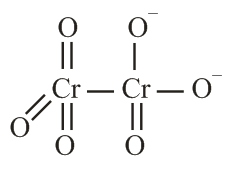
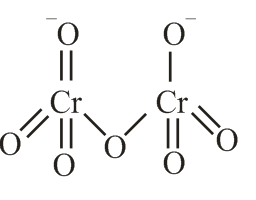
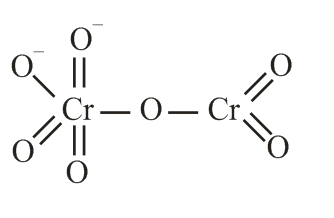
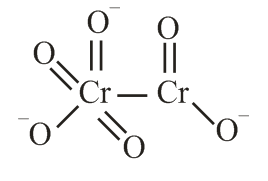
The correct structure of dichromate anion is

Important Points to Remember in Chapter -1 - The d- and f-Block Elements from Embibe Experts Gamma Question Bank for Engineering Chemistry Solutions
1. d-block elements & their Compounds:
The general electronic configuration of d-block elements is , where is the outermost shell. General trends in the chemistry of transition elements.
2. Metallic Character:
Nearly all the transition elements display typical metallic properties such as high tensile strength, ductility, malleability, high thermal, electrical conductivity, and metallic lustre. With the exceptions of and , they have one or more typical metallic structures at normal temperatures.
The transition elements (except for and ) are very much hard and have low volatility.
3. Melting and Boiling points:
The melting and boiling points of the transition series elements are generally very high.
4. Oxidation States:
Most transition elements show variable oxidation states. Participation of inner -electrons takes place in bonding in addition to outer ns-electrons because the energies of the and -subshells are nearly the same.
Different oxidation states of first transition series:
| Element |
Outer electronic configuration |
Oxidation states |
5. Characteristics of Oxides and Some Ions of and :
| O. S | Oxide / Hydroxide | Behaviour | Ion | Name of Ion | Colour of Ion |
| basic |
vanadium (vanadous) |
violet | |||
| basic |
Vanadium (vanadic) |
green | |||
| amphoteric |
oxovanadium (vanadyl) hypo vanadate (vanadite) |
blue brown |
|||
| amphoteric |
dioxovanadium orthovanadate |
yellow colourless |
|||
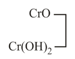 |
basic |
chromium (chromous) |
light blue | ||
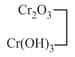 |
amphoteric | 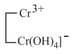 |
chromium chromic chromite |
violet green |
|
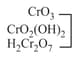 |
acidic | 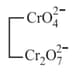 |
Chromate Dichromate |
yellow orange |
6. Standard electrode potentials:
The value of ionisation enthalpies gives information regarding the thermodynamic stability of the transition metal compounds in different oxidation states. Smaller the ionisation enthalpy of the metal, greater is the stability in its compound.
7. Electrode potentials:
In addition to ionisation enthalpy, the other factors such as enthalpy of sublimation, hydration enthalpy, ionisation enthalpy etc. determine the stability of a particular oxidation state in solution.
The overall energy change is:
The smaller the values of total energy change for a particular oxidation state in aqueous solution, greater will be the stability of that oxidation state. The electrode potentials are a measure of total energy change. Qualitatively, the stability of the transition metal ions in different oxidation states can be determined based on electrode potential data. The lower the electrode potential i.e., more negative the standard reduction potential of the electrode, the more stable is the oxidation state of the transition metal in the aqueous solution.
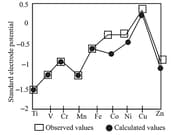
Thermochemical data for the first row Transition Elements and the Standard Electrode potentials for the Reduction of to
| Element | |||||
8. Formation of Coloured Ions:
Most of the compounds of transition metals are coloured in the solid form or solution form. The colour of the compounds of transition metals may be attributed to the presence of incomplete
-subshell.
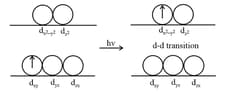
The excess of other colours constituting white light are transmitted and the compound appears coloured. The observed colour of a substance is always a complementary colour of the colour which is absorbed by the substance.
9. Magnetic Properties:
(i) Paramagnetic substances: The substances which are attracted by magnetic field are called paramagnetic substances.
(ii) Diamagnetic substances: The substances which are repelled by magnetic field are called diamagnetic substances. The ‘spin only’ magnetic moment can be calculated from the relation:
Where n is the number of unpaired electrons and is magnetic moment in Bohr magneton units. The paramagnetism first increases in any transition series and then decreases. The maximum Para magnetism is observed around the middle of the series (as it contains maximum number of unpaired electrons).
10. Formation of Interstitial Compounds:
Transition metals form interstitial compounds with elements such as hydrogen, boron, carbon, and nitrogen.
11. Catalytic properties:
Many transition metals and their compounds act as good catalysts for various reactions. Of these, the use of , etc. are very common.
(i) The catalytic property of transition metals is due to their tendency to form reaction intermediates with suitable reactants. These intermediates give reaction paths of lower activation energy and therefore, increase the rate of the reaction.
(ii) In some cases, the transition metal catalysts provide a suitable large surface area for the adsorption of the reactant. This increases the concentration of the reactants at the catalyst surface and weakens the bonds in the reactant molecules. Consequently, the activation energy gets lowered.
(iii) In some cases, the transition metal ions can change their oxidation states and become more effective as catalysts.
12. Alloy Formation:
Alloys are hard, have high melting points and are more resistant to corrosion than parent metals.
13. -block metal compounds:
(i) Hydrated Ferrous Sulphate , Ferric chloride and iron oxide .
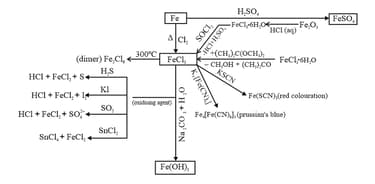
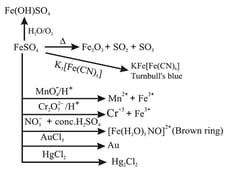
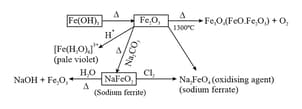
(ii) Hydrated copper sulphur
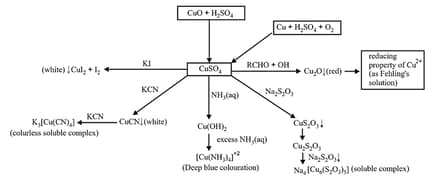
(iii) Silver nitrate
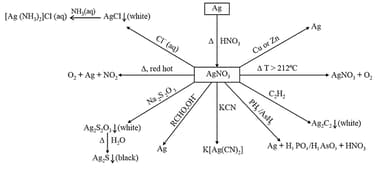
(iv) Potassium permanganate
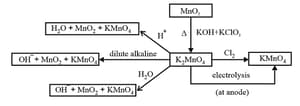
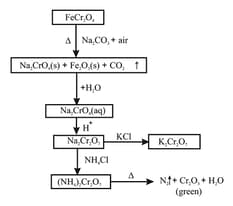
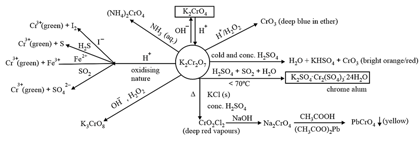
(v) Potassium dichromate