The correct structure of ethylenediaminetetraacetic acid is:
Important Points to Remember in Chapter -1 - Coordination Compounds from Embibe Experts Gamma Question Bank for Engineering Chemistry Solutions
1. Double Salts:
Those addition compounds which lose their identity in solutions.
2. Coordination Compounds:
Those addition compounds which retain their identity (i.e., does not lose their identity) in aqueous solution.
3. Central Atom/lons:
In a coordination entity-the atom/ion to which are bound a fixed number of ligands in a definite geometrical arrangement around it.
4. Ligands:
The neutral molecules, anions or cations which are directly linked with central metal atom or ion in the coordination entity are called ligands.
5. Chelate Ligand:
Chelate ligand is a di or polydentate ligand which uses its two or more donor atoms to bind a single metal ion producing a 5 or 6 membered ring.
6. Ambidentate Ligand:
Ligands which can donate through two different atoms present in it.
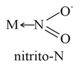
7. Coordination Number:
The number of ligand donor atoms to which the metal is directly attached.
8. Oxidation number of Central Atom:
The oxidation number of the central atom is defined as the charge it would carry if all the ligands are removed along with the electron pairs that are shared with the central atom.
9. Common monodentate and Ambidentate Ligands:
| Common Name | Name | Formula |
| Isocyanide methyl | methylisocyanide | |
| phosphine triphenyl | triphenylphosphine/triphenyl phosphine | |
| Pyridine | Pyridine | |
| Ammonia | Ammine | |
| Methyl amine | methylamine | |
| Water | aqua | |
| Carbonyl | Carbonyl | |
| Thiocarbonyl | thiocarbonyl | |
| Nitrosyl | Nitrosyl | |
| Fluoro | fluoro or fluorido | |
| Chloro | chloro or chlorido | |
| Bromo | bromo or bromido | |
| Iodo | iodo or iodido | |
| Cyano | cyanido or cyanido bonded) | |
| Isocyano | isocyanido or cyanido bonded) | |
| Thiocyano | thiocyanato- (-bonded) | |
| Isothiocyano | thiocyanato- (-bonded) | |
| cyanato (cyanate) | cyanato- (-bonded) | |
| Isocyanato (isocyanate) | cyanato- (-bonded) | |
| Hydroxo | hydroxo or hydroxido | |
| Nitro | nitrito- (-bonded) | |
| Nitrito | nitrito- (-bonded) | |
| Nitrate | Nitrato | |
| Amido | Amido | |
| Nitride | Nitrido | |
| Azido | Azido | |
| Hydride | Hydrido | |
| Oxide | Oxido | |
| Peroxide | Peroxido | |
| Superoxide | superoxido | |
| Acetate | Acetato | |
| Sulphate | Sulphato | |
| Thiosulphate | thiosulphato | |
| Sulphite | Sulphito | |
| hydrogen sulphite | hydrogensulphito | |
| Sulphide | sulphido or thio | |
| hydrogen sulphide | hydrogensulphido or mercapto | |
| Thionitrito | Thionitrito | |
| Nitrosylium | nitrosylium or nitrosonium | |
| Nitronium | nitronium |
10. Common Multidentate (Chelating) Ligands:
| Common name | Abbreviation | Formula | Structure |
| acetylacetonato | Acac | 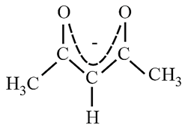 |
|
| -bipyridine | Bipy | 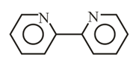 |
|
| Oxalato | Ox | 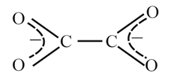 |
|
| Dimethylglyoximato | 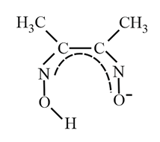 |
||
| Ethylenediaminetetraacetato | 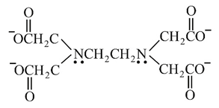 |
11. Homoleptic and heteroleptic complexes:
(i) Complexes in which a metal is bound to only one type of donor groups, e.g., are known as homoleptic.
(ii) Complexes in which a metal is bound to more than one type of donor groups, e.g., are known as heteroleptic.
12. Nomenclature of Coordination Compounds:
(i) Writing the formulas of Mononuclear Coordination Entities:
(a) The central atom is placed first.
(b) The ligands are then placed in alphabetical order. The placement of a ligand in the list does not depend on its charge.
(c) Polydentate ligands are also placed alphabetically. In case of abbreviated ligand, the first letter of the abbreviation is used to determine the position of the ligand in the alphabetical order.
(d) The formula for the entire coordination entity, whether charged or not, is enclosed in square brackets. When ligands are polyatomic, their formulas are enclosed in parentheses. Ligands abbreviations are also enclosed in parentheses.
(e) There should be no space between the ligands and the metal within a coordination sphere.
(f) The charge of the cation(s) is balanced by the charge of the anion(s).
(ii) Writing the name of Mononuclear Coordination Compounds:
(a) Like simple salts the cation is named first in both positively and negatively charged coordination entities.
(b) The ligands are named in alphabetical order (according to the name of ligand, not the prefix) before the name of the central atom/ion.
(c) Names of the anionic ligands end in and those of neutral ligands are the same except aqua for , ammine for , carbonyl for , thiocarbonyl for and nitrosyl for NO. But names of cationic ligands end in-ium.
(d) Prefixes mono, di, tri, etc., are used to indicate the number of the one kind of ligands in the coordination entity. When the names of the ligands include a numerical prefix or are complicated or whenever the use of normal prefixes creates some confusion, it is set off in parentheses and the second set of prefixes is used.
| Di | Bis | Tri | Tris | ||
| Tetra | Tetrakis | Penta | Pentakis | ||
| Hexa | Hexakis | Hepta | Heptakis |
(e) Oxidation state of the metal in cation, anion or neutral coordination entity is indicated by Roman numeral in the parentheses after the name of metal.
(f) If the complex ion is a cation, the metal is named same as the element. For example, in a complex cation is called cobalt and is called platinum. If the complex ion is an anion, the name of the metal ends with the suffix ate. For example, in a complex anion, is called cobaltite. For some metals, the Latin names are used in the complex anions.
| Iron | ferrate | lead | plumbate |
| silver | argentate | tin | stannate |
| gold | aurate |
(g) The neutral complex molecule is named like that of the complex cation.
13. Theories in coordination compounds:
(i) Werner's Theory:
According to Werner most elements exhibit two types of valencies: Primary valency and Secondary valency.
(a) Primary valency:
This corresponds to oxidation state of the metal ion. This is also called principal, ionizable or ionic valency. It is satisfied by negative ions and its attachment with the central metal ion is shown by dotted lines.
(b) Secondary or auxiliary valency:
It is also termed as coordination number (usually abbreviated as ) of the central metal ion. It is non-ionic or non-ionizable (i.e., coordinate covalent bond type). In the modem terminology, such spatial arrangements are called coordination polyhedra and various possibilities are:
| Linear | ||
| Triangular | ||
| tetrahedral or square planar | ||
| octahedral |
14. Effective Atomic Number Rule given by Sidgwick:
Effective Atomic Number Atomic no. of central metal Oxidation state of central metal No. of electrons donated by ligands.
15. Valence bond theory:
The model utilizes hybridization of or orbitals of metal atom or ion to yield a set of equivalent orbitals of definite geometry to account for the observed structures such as octahedral, square planar and tetrahedral, and magnetic properties of complexes. The number of unpaired electrons measured by the magnetic moment of the compounds determines which -orbitals are used.
16. Coordination Number Six:
In the diamagnetic octahedral complex, the cobalt ion is in oxidation state and has the electronic configuration represented as shown below.

If the complex is paramagnetic and uses outer orbital in hybridization; it is thus called as outer orbital or high spin or spin free complex. So,

17. Coordination Number Four:
In the paramagnetic and tetrahedral complex, the nickel is in oxidation state and the ion have the electronic configuration. The hybridization scheme is as shown in figure.
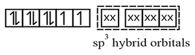
18. It suffers from the following shortcomings:
(i) Several assumptions are involved.
(ii) There is no quantitative interpretation of magnetic data.
(iii) It has nothing to say about the spectral (color) properties of coordination compounds.
(iv) It does not give a quantitative interpretation of the thermodynamic or kinetic stabilities of coordination compounds.
(v) It does not make exact predictions regarding the tetrahedral and square-planar structures of -coordinate complexes.
(vi) It does not distinguish between strong and weak ligands.
19. Magnetic Properties of Coordination Compounds:
Magnetic moment
Units of Magnetic Moment Bohr Magneton; number of unpaired electrons
For metal ions with upto three electrons in the -orbitals like; two vacant d-orbitals are easily available for octahedral hybridization. The magnetic behavior of these free ions and their coordination entities is similar. When more than three electrons are present, like in and the required two vacant orbitals for hybridization is not directly available (because of Hund's rules). Thus, for cases, two vacant d orbitals are only available for hybridization because of paining of electrons which leaves two, one and zero unpaired electrons, respectively.
20. Crystal Field Theory:
The crystal field theory is an electrostatic model which considers the metal-ligand bond to be ionic arising purely from electrostatic interaction between the metal ion and the ligand.
(a) Crystal field splitting in octahedral coordination entities:
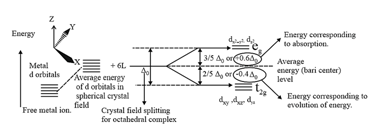
The crystal field splitting depends upon the fields produced by the ligand and charge on the metal ion.
Ligands can be arranged in a series in the orders of increasing field strength as given below:
Such a series is termed as spectrochemical series. It is an experimentally determined series based on the absorption of light by complexes with different ligands.
21. Calculation of Crystal Field Stabilization energy :
(i) Formula:
where are number of electron orbitals respectively and crystal field splitting energy for octahedral complex. represents the number of extra electron pairs formed because of the ligands in comparison to normal degenerate configuration.
(ii) Crystal field splitting in tetrahedral coordination entities:
In tetrahedral coordination entity formation, the d orbital splitting is inverted and is smaller as compared to the octahedral field splitting. For the same metal, the same ligands, and metal-ligand distances, it can be shown that:
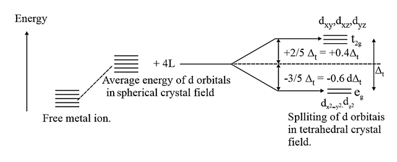
Figure showing crystal field splitting in tetrahedral complex.
22. Color of Coordination compounds:
According to the crystal field theory the color is due to the transition of electron under the influence of ligands. We know that the color of a substance is due to the absorption of light at a specific wavelength in the visible part of the electromagnetic spectrum ( to ) and transmission or reflection of the rest of the wavelengths.
23. Limitations of Crystal Field Theory:
(i) It considers only the metal ion -orbitals and gives no consideration at all to other metal orbitals (such as orbitals)
(ii) It is unable to account satisfactorily for the relative strengths of ligands. For example, it gives no explanation as to why is a stronger ligand than in the spectrochemical series.
(iii) According to this theory, the bond between the metal and ligands are purely ionic. It gives no account on the partly covalent nature of the metal ligand bonds.
(iv) The cannot account for the in complexes.
24. Stability of Coordination Compounds:
The stability of a coordination compound is measured in terms of the stability constant (equilibrium constant) given by the expression for the overall reaction:
Overall equiulibrium constant for formation of complex
Greater is the value of , greater is the stability of the complex.
25. Factor affecting splitting:
(i) Strength of ligand
(ii) Oxidation state of central metal lon
(iii) Transition series (-series)
(iv) Geometry (number of ligands).
26. Strength of Ligand depends upon:
(i) good donor.
(ii) good acceptor.
(iii) high negative charge.
(iv) Small in size.
27. Series which shows the relative strength of Ligands:
(i)
(ii) Complementary color relationship: The color absorbed is complementary to the color emitted.
For example: Complementary color of red is green.
28. Structural Isomerism:
(i) Ionization isomerism: Counter lon acts as a ligand & ligand act as counter ion.
(ii) Hydrate isomerism: Number of water molecule inside & outside the coordination spheres different.
(iii) Linkage: when ambidentate ligand is present in coordination sphere.
(iv) Co-ordination isomerism: In cationic anionic complex when ligand/metal lon interchange:
29. Stereoisomerism:
(i) Geometrical isomerism:
(a) Tetrahedral does not show geometrical Isomerism.
(b) Square planar , Show geometrical isomerism.
(c) have geometrical isomerism.
(d) have geometrical isomerism [fac-mer]
(ii) Optical isomerism: (does not have plane of symmetry)
(a) Tetrahedral is optically active.
(b) Generally square planar is not optically active but in some cases, it becomes optically active due to rotation of the compound and due to absence of Plane of symmetry in the molecule.
(c) is optically active.
(d) cis is optically active but trans is not optically active.
(e) is optically active.
