MEDIUM
Earn 100
The decreasing order of bond enthalpies of the following alkyl halides is:
(i)
(ii)
(iii)
(iv)
(a)
(b)
(c)
(d)
73.11% studentsanswered this correctly
Important Questions on Haloalkanes and Haloarenes
MEDIUM
MEDIUM
MEDIUM
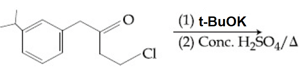
HARD
EASY
EASY
(A)
(B)
(C)
(D)
EASY
This reaction will be the fastest in
EASY
MEDIUM
MEDIUM
MEDIUM
EASY
EASY
MEDIUM
EASY
HARD
EASY
MEDIUM
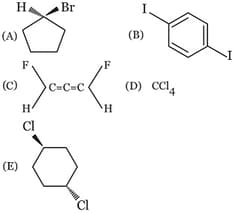
MEDIUM


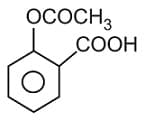 is used as:
is used as: