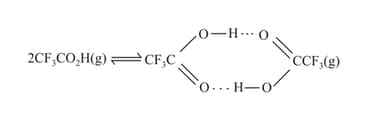
The density of trifluoroacetic acid vapour was determined at and and was found to be . Calculate for:


Important Questions on Equilibrium
If the reaction between steam and iron proceeds as
and partial pressures of steam and hydrogen are and , respectively at . Calculate the partial pressure of steam at equilibrium when partial pressure of hydrogen is .
Answer correct up to one place of decimal.
A chemist needs a buffer with How many of pure acetic acid (density ) must be added to of solution to obtain such a buffer?
Give answer after multiplying with 10 and rounding off to the nearest integer value.
A quantity of is added to a solution containing mole of acetic acid. The final volume of the solution is and the of the solution is .
What was the original concentration of the acetic acid?
Round off your to the nearest integer.
The reaction:
was studied by analysing the equilibrium mixture for the amount of produced. A vessel whose volume was was filled with mole of and mole of . After the mixture came to equilibrium in the closed vessel at the gaseous mixture was removed, and the was dissolved in water. Sufficient ions were added to react completely with the to precipitate . If of was obtained, what is the value of at
Give answer after multiplying with 100 and rounding off to the nearest integer value.
The following equilibrium exists in a closed system at .
When a sample of pure is placed in an evacuated vessel and allowed to reach equilibrium at , the total pressure is . Find the value of . (Give answer after multiplying with 100 and rounding off to the nearest integer value.)
