The electron and the neutron belong to different groups of particles.
Copy and complete the table to show the group of particles to which the electron and neutron belong and state the name of another member of each group.
Group to which it belongs
Another particle in the same group
Electron
Neutron

Important Questions on Atomic Structure and Particle Physics
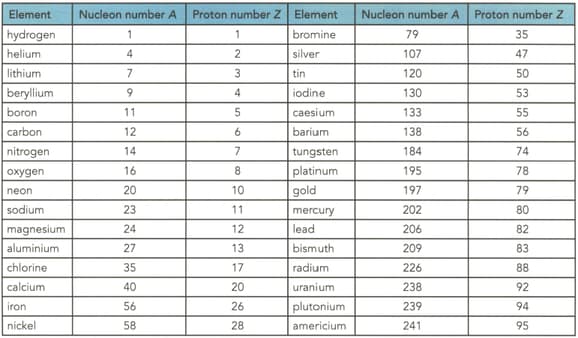
Table shows the proton and nucleon numbers of several nuclei. Determine the number of neutrons in the nuclei of the following elements shown in the table
(a) Nitrogen
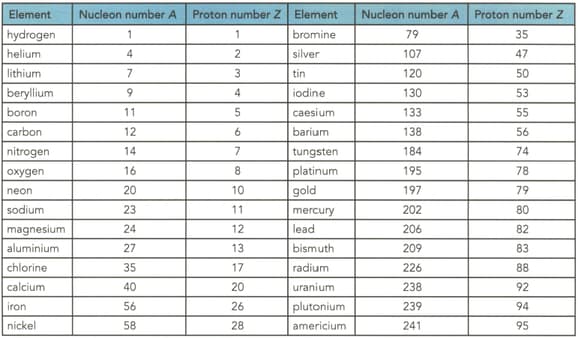
Table shows the proton and nucleon numbers of several nuclei. Determine the number of neutrons in the nuclei of the following elements shown in the table
(b) Bromine
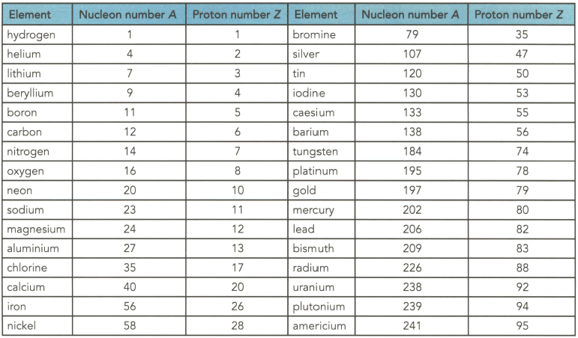
Table shows the proton and nucleon numbers of several nuclei. Determine the number of neutrons in the nuclei of the following elements shown in the table
(c) Silver
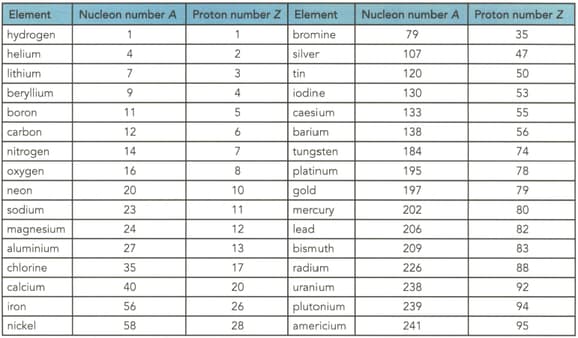
Table shows the proton and nucleon numbers of several nuclei. Determine the number of neutrons in the nuclei of the following elements shown in the table
(d) Gold
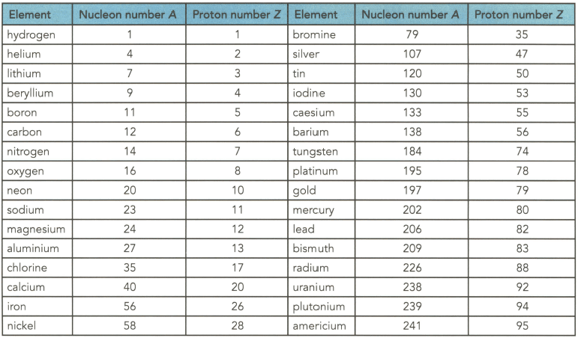
Table shows the proton and nucleon numbers of several nuclei. Determine the number of neutrons in the nuclei of the following elements shown in the table
(e) Mercury
State the charge of each of the following in terms of the elementary charge
(c) Nucleus
