The element germanium is in Group 14, in Period 4. It is classed as a semimetal or metalloid, as is silicon in Period 3.
Predict the chemical bonding and structure of the element germanium.

Important Questions on Periodicity
The element germanium is in Group 14, in Period 4. It is classed as a semimetal or metalloid, as is silicon in Period 3.
Germanium(IV) oxide has properties similar to silicon dioxide. It is an acidic oxide. Write a balanced symbol equation, including state symbols, to show the reaction of germanium(IV) oxide with hot, concentrated sodium hydroxide solution.
The element germanium is in Group 14, in Period 4. It is classed as a semimetal or metalloid, as is silicon in Period 3.
What would you expect to happen if germanium(IV) oxide was added to hydrochloric acid?
i) Does element X belong to Group 1, Group 2 or Group 15 of the Periodic Table?
ii) What type of reaction takes place between X and water?
iii) Identify the white fumes given off when X reacts with water.
The graph shows how a periodic property varies when plotted against atomic number for Period 3 (sodium to argon).
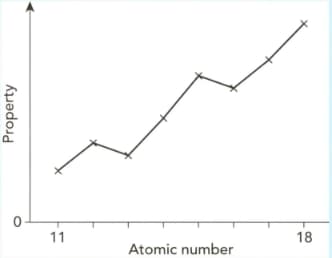
(i) Identify the property.
(ii) Explain the overall trend across the period.
ii) Explain this trend.
