EASY
JEE Main/Advance
IMPORTANT
Earn 100
The enthalpy change for a certain reaction at is The entropy change under these conditions is The free energy change for the reaction and its spontaneous/non-spontaneous character will be:
(a) spontaneous
(b) non-spontaneous
(c) spontaneous
(d)None of these
100% studentsanswered this correctly

Important Questions on Thermodynamics
MEDIUM
JEE Main/Advance
IMPORTANT
Look at the following diagram:
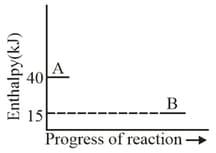
The enthalpy change for the reaction will be
MEDIUM
JEE Main/Advance
IMPORTANT
In Haber's process of manufacturing of ammonia:
| Molecule | |||
If is independent of temperature, then reaction at as compared to that of will be:
HARD
JEE Main/Advance
IMPORTANT
HARD
JEE Main/Advance
IMPORTANT
The heat of formation of is . The heat of combustion of is . for and are and respectively. Then, the for the isomerisation reaction and for the same are at
EASY
JEE Main/Advance
IMPORTANT
HARD
JEE Main/Advance
IMPORTANT
EASY
JEE Main/Advance
IMPORTANT
MEDIUM
JEE Main/Advance
IMPORTANT
