The enthalpy change of a reaction is independent of
Important Questions on Thermodynamics
fish swimming in water body when taken out from the water body is covered with a film of water of weight . When it is subjected to cooking at , then the internal energy for vaporization in is integer]
[Assume steam to be an ideal gas. Given for water at and bar is ]
For which of the following systems, the difference between and is not significant?
(i) Solids
(ii) Gases
(iii) Mixture of gases and liquids
(iv) Liquids
The internal energy change (in ) when of water undergoes complete evaporation at is ..............
(Given : for water at , )
Calculate the work done in the following reaction at . State whether work is done on the system or by the system.
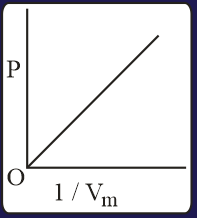
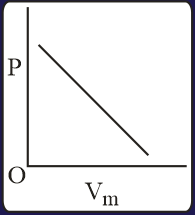
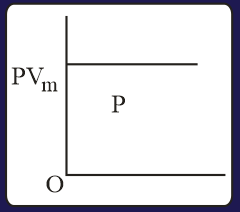
The combination of plots which does not represent isothermal expansion of an ideal gas is