The enthalpy of combustion of methanol can also be determined experimentally in a school laboratory. A burner containing methanol was weighed and used to heat water in a test tube as illustrated in figure.
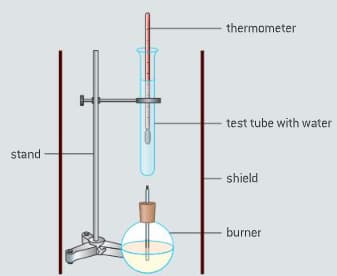
The data shown in table were collected.
Initial mass of burner and methanol /
Final mass of burner and methanol /
Mass of water in test tube /
Initial temperature of water /
Final temperature of water
Determine the enthalpy change, in , for the combustion of one mole of methanol.


Important Questions on Energetics and Thermochemistry
Methanol is made in large quantities as it is used in the production of polymers and in fuels. The enthalpy of combustion of methanol can be determined theoretically or experimentally .
Using the data from the given table, the value of enthalpy of combustion of methanol is found to be.
| Bond | ||||||
| Average bond enthalpy () |
The data booklet value for the enthalpy of combustion of methanol is . Suggest why this value differs from the values calculated theoretically.
The enthalpy of combustion of methanol can also be determined experimentally in a school laboratory. A burner containing methanol was weighed and used to heat water in a test tube as illustrated in figure.
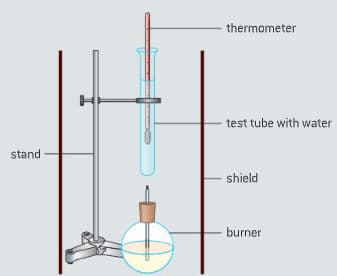
The data shown in table were collected.
| Initial mass of burner and methanol / | |
| Final mass of burner and methanol / | |
| Mass of water in test tube / | |
| Initial temperature of water / | |
| Final temperature of water |
The data booklet value for the enthalpy of combustion of methanol is . Suggest why this value differs from the values calculated experimentally.
