The equivalent conductances of two strong electrolytes at infinite dilution in (where ions move freely through a solution) at are given below
equiv
equiv
What additional information/quantity one needs to calculate of an aqueous solution of acetic acid?
equiv
Important Questions on Electrochemistry
Match List - I with List - II :
| List - I (Parameter) |
List - II (Unit) | ||
| (a) | Cell constant | (i) | |
| (b) | Molar conductivity | (ii) | Dimensionless |
| (c) | Conductivity | (iii) | |
| (d) | Degree of dissociation of electrolyte | (iv) |
(where the constant B is positive)
Given below are two statements:
Statement I : The limiting molar conductivity of (strong electrolyte) is higher compared to that of $\mathrm{CH}_{3} \mathrm{COOH}$ (weak electrolyte).
Statement II : Molar conductivity decreases with decrease in concentration of electrolyte.
In the light of the above statements, choose the most appropriate answer from the options given below:
The molar conductivity of a conductivity cell filled with moles of solution is and that of moles another identical cell heaving solution is , The conductivities exhibited by these two cells are same.
The relationship between and is
Given below are two statements:
Statement I: For , molar conductivity increases steeply with dilution.
Statement II: For carbonic acid, molar conductivity increases slowly with dilution. In the light of the above statements, choose the correct answer from the options given below
The variation of molar conductively with concentration of an electrolyte (X) in aqueous solution is shown in the given figure.
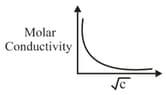
The electrolyte X is :
: Conductivity always increases with decreases in the concentration of electrolyte.
: Molar conductivity always increases with decreases in the concentration of electrolyte.
The correct option among the following

