HARD
JEE Advanced
IMPORTANT
Earn 100
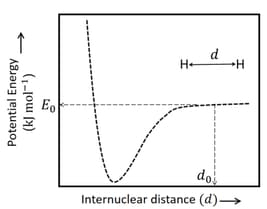
The figure below is the plot of potential energy versus internuclear distance of molecule in the electronic ground state. The value of the net potential energy (as indicated in the figure) is for at which the electron-electron repulsion and the nucleus-nucleus repulsion energies are absent, find the value of to the nearest integer value.
As reference, the potential energy of atom is taken as zero when its electron and the nucleus are infinitely far apart.
Use Avogadro constant as Give an answer to the nearest integer value.

50% studentsanswered this correctly

Important Questions on Structure of Atom
HARD
JEE Advanced
IMPORTANT
The ground state energy of hydrogen atom is . Consider an electronic state of whose energy, azimuthal quantum number and magnetic quantum number are and respectively. Which of the following statement(s) is(are) true for the state ?
MEDIUM
JEE Advanced
IMPORTANT
Consider the Bohr's model of a one-electron atom where the electron moves around the nucleus. In the following List-I contains some quantities for the orbit of the atom and List-II contains options showing how they depend on .
| List - I | List - II |
|---|---|
| (I) Radius of the orbit | |
| (II) Angular momentum of the electron in the orbit | |
| (III) Kinetic energy of the electron in the orbit | |
| (IV) Potential energy of the electron in the orbit | |
Which of the following options has the correct combination considering List-I and List-II?
HARD
JEE Advanced
IMPORTANT
Consider the Bohr's model of a one-electron atom where the electron moves around the nucleus. In the following List-I contains some quantities for the orbit of the atom and List-II contains options showing how they depend on .
| List - I | List - II |
|---|---|
| (I) Radius of the orbit | |
| (II) Angular momentum of the electron in the orbit | |
| (III) Kinetic energy of the electron in the orbit | |
| (IV) Potential energy of the electron in the orbit | |
Which of the following options has the correct combination considering List-I and List-II?
HARD
JEE Advanced
IMPORTANT
Among the species given below, the total number of diamagnetic species is ___.
(superoxide), dimeric sulphur in vapour phase,
HARD
JEE Advanced
IMPORTANT
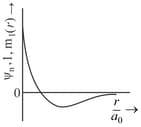
The wave function, is a mathematical function whose value depends upon spherical polar coordinates of the electron and characterized by the quantum numbers and . Here is distance from nucleus, is colatitude and is azimuth. In the mathematical functions given in the Table, Z is atomic number and is Bohr radius.
| Column – 1 | Column – 2 | Column – 3 |
| (I) orbital | (i) |
(P) |
| (II) orbital | (ii) One radial node | (Q) Probability density at nucleus |
| (III) orbital | (iii) | (R) Probability density is maximum at nucleus |
| (IV) orbital | (iv) -plane is a nodal plane | (S) Energy needed to excite an electron from state to state is times the energy needed to excite electron from state to state. |
HARD
JEE Advanced
IMPORTANT
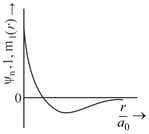
The wave function, is a mathematical function whose value depends upon spherical polar coordinates of the electron and characterized by the quantum numbers and . Here is the distance from the nucleus, is colatitude and is azimuth. In the mathematical functions given in the Table, is the atomic number and is Bohr radius
| Column 1 | Column 2 | Column 3 |
| (I) orbital | (i) |
(P) |
| (II) orbital | (ii) One radial node | (Q) Probability density at the nucleus . |
| (III) orbital | (iii) | (R) Probability density is maximum at the nucleus. |
| (IV) orbital | (iv) -plane is a nodal plane | (S) Energy needed to excite an electron from state to state is times the energy needed to excite are electron from state to state. |
HARD
JEE Advanced
IMPORTANT
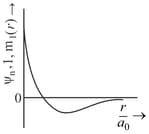
The wave function, is a mathematical function whose value depends upon spherical polar coordinates of the electron and characterized by the quantum numbers and . Here is the distance from the nucleus, is colatitude and is azimuth. In the mathematical functions given in the Table, is the atomic number and is Bohr radius
| Column 1 | Column 2 | Column 3 |
| (I) orbital | (i) |
(P) |
| (II) orbital | (ii) One radial node | (Q) Probability density at the nucleus . |
| (III) orbital | (iii) | (R) Probability density is maximum at the nucleus. |
| (IV) orbital | (iv) -plane is a nodal plane | (S) Energy needed to excite an electron from state to state is times the energy needed to excite are electron from state to state. |
MEDIUM
JEE Advanced
IMPORTANT
is the probability of finding the electron of the hydrogen atom in a spherical shell of infinitesimal thickness, , at a distance, , from the nucleus. The volume of this shell is . The qualitative sketch of the dependence of on is: