HARD
AS and A Level
IMPORTANT
Earn 100
The first ionization energies of four consecutive elements in the periodic table are :
Sodium =
Magnesium=
Aluminium =
Silicon=
Explain why aluminium has lower first ionization energy than magnesium.

Important Questions on Electrons in Atoms
MEDIUM
AS and A Level
IMPORTANT
The ionization energy of fluorine is whereas the first ionization energy of Iodine is . Explain why fluorine has higher first ionization energy than Iodine despite fluorine having a smaller nuclear charge.
MEDIUM
AS and A Level
IMPORTANT
The sketch graph shows the successive ionization energies of aluminium.
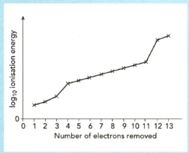
Define the term first ionization energy.
MEDIUM
AS and A Level
IMPORTANT
The sketch graph shows the successive ionization energies of aluminium.
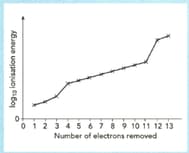
Explain how the graph provides evidence for the existence of three electrons shells in an aluminium atom.
MEDIUM
AS and A Level
IMPORTANT
Write an equation, including state symbols, to represent the ionization energy aluminium.
HARD
AS and A Level
IMPORTANT
Write the electronic configuration of an aluminium ion, using notation.
MEDIUM
AS and A Level
IMPORTANT
The table shows the first ionization energies,,in , of the elements in the period of the periodic table.
| Element | Na | Mg | Al | Si | P | S | Cl | Ar |
| 494 | 736 | 577 | 786 | 1060 | 1000 | 1260 | 1520 |
Explain why there is a general increase in the value of across the period.
MEDIUM
AS and A Level
IMPORTANT
The table shows the first ionization energies,,in , of the elements in the period of the periodic table.
| Element | Na | Mg | Al | Si | P | S | Cl | Ar |
| 494 | 736 | 577 | 786 | 1060 | 1000 | 1260 | 1520 |
Explain why aluminium has a lower value of than magnesium.
MEDIUM
AS and A Level
IMPORTANT
The table shows the first ionization energies,,in , of the elements in the period of the periodic table.
| Element | Na | Mg | Al | Si | P | S | Cl | Ar |
| 494 | 736 | 577 | 786 | 1060 | 1000 | 1260 | 1520 |
Write the electronic configuration for argon using notation.
