EASY
JEE Main
IMPORTANT
Earn 100
The first law of thermodynamics is a special case of,
(a)Newton's law.
(b)law of conservation of energy.
(c)Charles' law.
(d)law of heat exchange.
97.44% studentsanswered this correctly

Important Questions on Thermodynamics
EASY
JEE Main
IMPORTANT
EASY
JEE Main
IMPORTANT
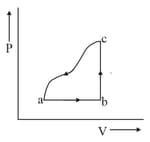
A given quantity of gas is taken from the state to the state reversibly by two paths, directly and as shown in the figure below:
During the process , work done by the gas is and heat absorbed is . If during the process , the work done by the gas is , the heat absorbed is
EASY
JEE Main
IMPORTANT
EASY
JEE Main
IMPORTANT
EASY
JEE Main
IMPORTANT
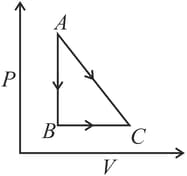
Find the work done by the gas in the process shown in figure.
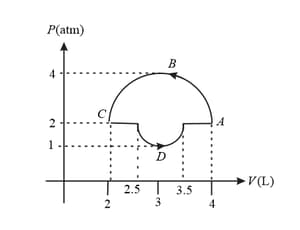
EASY
JEE Main
IMPORTANT
EASY
JEE Main
IMPORTANT
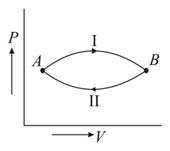
A gas at state changes to state through path and shown in figure. The change in internal energy is and , respectively. Then
EASY
JEE Main
IMPORTANT
