EASY
Earn 100
The following compound can exhibits
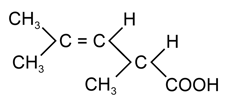
(a)Tautomerism
(b)Optical isomerism
(c)Geometrical isomerism
(d)Geometrical and optical isomerism
50% studentsanswered this correctly
Important Questions on Coordination Compounds
EASY
MEDIUM
MEDIUM
HARD
(i) Two isomers are produced if the reactant complex ion is a cis-isomer.
(ii) Two isomers are produced if the reactant complex ion is a trans-isomer.
(iii) Only one isomer is produced if the reactant complex ion is a trans-isomer.
(iv) Only one isomer is produced if the reactant complex ion is a cis-isomer.
The correct statements are
HARD
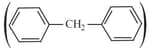 is .
is .How many structural isomers are possible when one of the hydrogens is replaced by a chlorine atom?
MEDIUM
HARD
(a)
(b) and
(c) is:
MEDIUM
MEDIUM
HARD
MEDIUM
MEDIUM
MEDIUM
MEDIUM
MEDIUM
(ethanediamine)
HARD
MEDIUM
EASY
MEDIUM
HARD

