MEDIUM
Earn 100
The following graph represents the titration curve for a diprotic acid using a standardized solution of sodium hydroxide. Which one of the following statements is correct ?
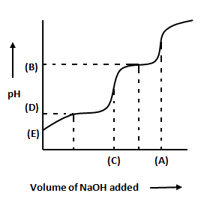
(a)The volume (A) represents the volume for the first equivalence point.
(b)The pH (B) represents the end point at .
(c)The volume (C) represents complete neutralization for the diprotic acid.
(d)The pH (D) represents the half - neutralization pH that may be used to estimate .
50% studentsanswered this correctly
Important Questions on Equilibrium
MEDIUM
(First dissociation constant of density of the soft drink )
EASY
HARD
HARD
MEDIUM
EASY
(A)
(B)
(C)
(D)
MEDIUM
HARD
The pH of a mixture containing of and of will be approximately
Ionic product of water is temperature dependent.
A monobasic acid with has a . The degree of dissociation of this acid is .
The Le Chatelier's principle is not applicable to common-ion effect.
The correct statements are:
MEDIUM
MEDIUM
(i)
(ii)
(iii)
(iv)
The of which one of them will be equal to ?
EASY
Two solutions and have and respectively. Which of the following statement is correct ?
MEDIUM
MEDIUM
MEDIUM
MEDIUM
EASY
EASY
MEDIUM

