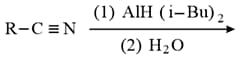MEDIUM
Earn 100
The formation of aldehyde from alkyl cyanide is related with the name
(a)Stephen
(b)Rosenmund
(c)Wurtz
(d)HVZ reaction
50% studentsanswered this correctly
Important Questions on Aldehydes, Ketones and Carboxylic Acids
EASY
Identify compound (A) in the following reaction :
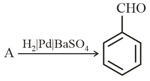
HARD
A substance , yields on oxidation a compound, which gives an oxime and a positive iodoform test. The original substance on treatment with concentrated gives The structure of the compound is
MEDIUM
An alkene on ozonolysis gives methanal as one of the product. Its structure is
MEDIUM
The major product of the following reaction sequence is
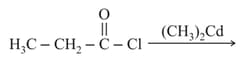
HARD
In the following reaction is
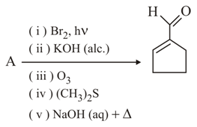
MEDIUM
Reductive ozonolysis of followed by hydrolysis gives
HARD
The major product obtained from the following reaction is :
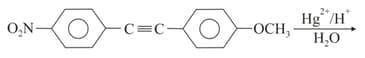
EASY
The reaction of acetylene with , in the presence of a catalytic amount of and dilute , gives the product . Compound , upon treatment with aqueous , forms . What are and ?
HARD
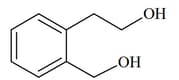
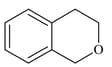
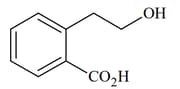
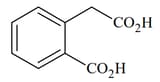
List includes starting materials and reagents of selected chemical reactions. Listgives structures of compounds that may be formed as intermediate products and/or final products from the reactions of List.
| List-I | List-II |
|---|---|
(I) 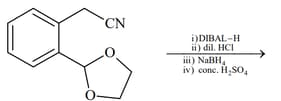 |
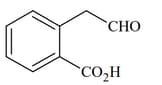 |
(II) 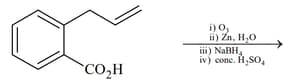 |
 |
(III) 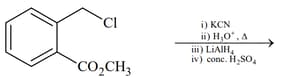 |
 |
(IV) 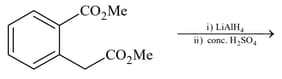 |
 |
 |
|
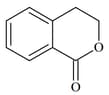 |
Which of the following options has the correct combination considering List and List?
MEDIUM
The correct match between Item-I (starting material) and Item-II (reagent) for the preparation of benzaldehyde is :
| Item-I | Item-II | ||
| (I) | Benzene | (P) | and |
| (II) | Benzonitrile | (Q) | and quinoline |
| (III) | Benzoyl Chloride | (R) |
MEDIUM
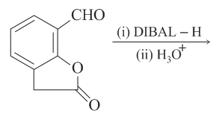
The major product of the following reaction is:
MEDIUM
The major product obtained in the following reaction is
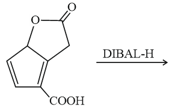
MEDIUM
The major product obtained in the reaction of isobutyl benzene with acetic anhydride in the presence of anhydrous is:
MEDIUM
An alkene of molecular formula , on ozonolysis gives dimethylpropanal & butanone, then the alkene is
MEDIUM
Which of the following is obtained by hydrogenation of benzoyl chloride in presence of on ?
EASY
Which of the following is Rosenmund reduction?
MEDIUM
What are the products formed in the reaction given below:
?
HARD
Consider the following reactions:
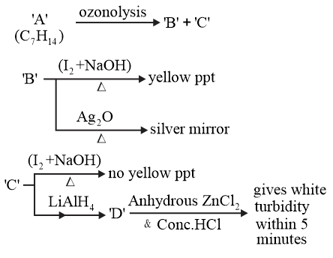
'A' is
MEDIUM
An alkene on reaction with and gives propanone and ethanal in equimolar ratio. Additional of to alkene gives as the major product. The structure of product is:
EASY
The major product of the following reaction is,