The formula for calcium oxide is:
Important Questions on Atoms and Molecules
type of semi-conductor material
amount of doping
temperature
Which one of the following is correct?
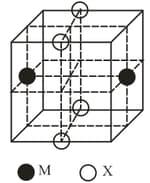
Remove all the anions , except the central one.
Replace all the face centered cations , by anions .
Remove all the corner cations .
Replace the central anion , with cation .
The value of in is _____.
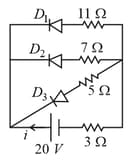
In the following circuit diagram, the current through the battery is
The cubic unit cell structure of a compound containing cation and anion is shown below. When compared to the anion, the cation has smaller ionic radius. Choose the correct statement(s).
Match the following
List - I List-II
A) Metallic solid I) Carbon tetrachloride
B) Covalent solid II) Calcium fluoride
C) Molecular solid III) Copper
D) Ionic solid IV) Silicon carbide
V) Glass
The correct answer is
A solution of a substance 'X' is used for whitewashing.
(a) Name the substance 'X' and write its formula.
(b) Write the reaction of the substance with water.

