MEDIUM
Earn 100
The gas liberated by the electrolysis of dipotassium succinate solution is:
(a)Ethyne
(b)Propene
(c)Ethene
(d)Ethane
50% studentsanswered this correctly
Important Questions on Unsaturated Hydrocarbons- Alkenes
HARD
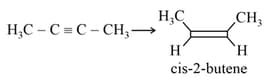
EASY
MEDIUM
HARD
The major product in the following sequence of reaction is :
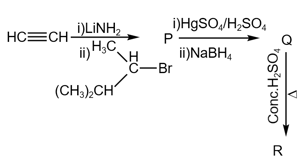
HARD
EASY
HARD
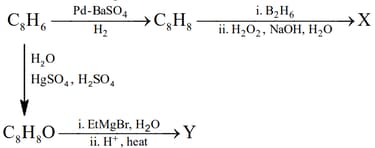
Compound X is
EASY
HARD
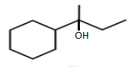 ?
?MEDIUM
HARD
Consider the following transformations of a compound .
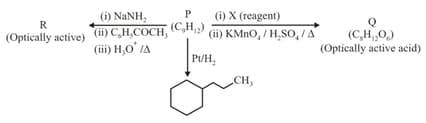
Choose the correct option(s).
EASY
In the reaction
EASY
MEDIUM
Consider the following reactions:
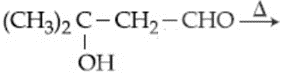
Which of the reaction(s) will not produce Saytzeff product?
EASY
EASY
The reagent used for the following reaction is
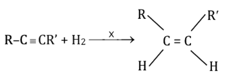
EASY
HARD
EASY
The major product of the following reaction is:
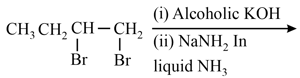
EASY


