EASY
NEET
IMPORTANT
Earn 100
The graph plotted between concentration versus time
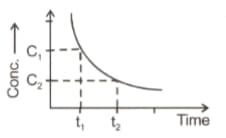
(a) It gives rate of disappearance of reactant
(b)Rate $=-\frac{d\left[C_{2}-C_{1}\right]}{t_{2}-t_{1}}$
(c)Both (1) \& (2)
(d)It predicts the order of reaction
25.42% studentsanswered this correctly

Important Questions on Chemical Kinetics
EASY
NEET
IMPORTANT
Which of the following is correct?
MEDIUM
NEET
IMPORTANT
EASY
NEET
IMPORTANT
The rate law of a reaction between the substances and is given by, rate . On doubling the concentration of $A$ and making the volume of half the ratio of new rate to the earlier rate of reaction will be
EASY
NEET
IMPORTANT
EASY
NEET
IMPORTANT
EASY
NEET
IMPORTANT
Assuming an elementary reaction . The effect on the rate of this reaction brought about by doubling the concentration of without changing the order
EASY
NEET
IMPORTANT
EASY
NEET
IMPORTANT
The late law for the reaction
is given by Rate . The rate of reaction will be
