MEDIUM
AS and A Level
IMPORTANT
Earn 100
The graph shows a sketch of (ionization energy) against number of electrons removed for magnesium.
Use this graph to answer the following questions.
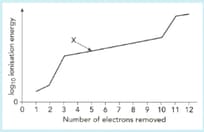
Explain what information the graph gives about the electron arrangement of magnesium.

Important Questions on Electrons in Atoms
MEDIUM
AS and A Level
IMPORTANT
Why must the inside of an electron microscope be at very low pressure so that there is hardly any air present ?
HARD
AS and A Level
IMPORTANT
For the element aluminium(), Sketch a graph to predict the of the successive ionization energies (Y-axis) against the number of electrons removed (X-axis).
HARD
AS and A Level
IMPORTANT
Sketch a graph to show the values of the first four ionization energies of a group element.
HARD
AS and A Level
IMPORTANT
Which block in the periodic table does the element with the electronic configuration belong to ?
MEDIUM
AS and A Level
IMPORTANT
The graph shows a sketch of (ionization energy) against number of electrons removed for magnesium.
Use this graph to answer the following questions.
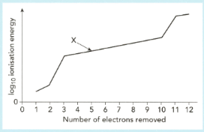
Construct the equation for the ionization energy marked (the ionization energy).
MEDIUM
AS and A Level
IMPORTANT
Define the term first ionization energy.
MEDIUM
AS and A Level
IMPORTANT
Sketch a graph to show how (Ionization energies for chlorine (atomic number ) varies when the plotted against number of electron removed.
MEDIUM
AS and A Level
IMPORTANT
Explain the shape of the graph you have drawn for (ionization energy) for Chlorine varies when plotted against number of electron removed.
